views
Clinical Trial Supplies Market in United States 2024:
How Big is the United States Clinical Trial Supplies Industry?
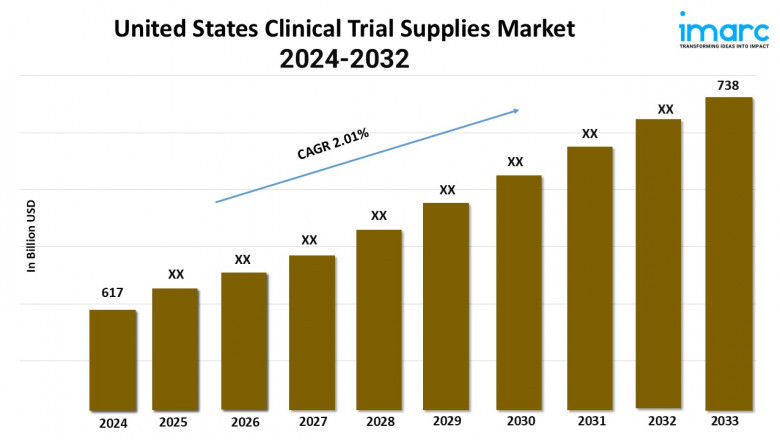
The United States clinical trial supplies market size reached USD 617 Million in 2023. Looking forward, IMARC Group expects the market to reach USD 738 Million by 2032, exhibiting a growth rate (CAGR) of 2.01% during 2024-2032.
Base Year: 2023
Historical Years: 2018-2023
Forecast Years: 2024-2032
Market Size in 2023: USD 617 Million
Market Size in 2032: USD 738 Million
Market Growth Rate (CAGR) 2024-2032: 2.01%
United States Clinical Trial Supplies Market Trends and Drivers:
The United States clinical trial supplies market is also experiencing robust growth driven by intersection of technological, regulatory, and operational forces that are transforming the character of clinical trials and how clinical trials are being facilitated in the health care industry. Essentially, the increasing sophistication of clinical trials and the growing need for innovative treatment are pushing sponsors to invest a lot of money in innovative, efficient, and scalable supply solutions that guarantee timely and effective delivery of study materials. In addition, the increasingly rapid pace of medical advances, including advances in biotechnology and personalized medicine, has put a growing burden on specialized technology, from research chemicals and lab reagents to equipment and information gathering hardware. Also, increased control by regulatory bodies and enhanced quality standards by regulatory bodies such as the FDA are compelling companies to introduce more stringent supply chain practices in a way where clinical trial supplies are compliant, secure, and traceable end-to-end throughout the trial life cycle. Besides, fast technology growth in fields such as automation, real-time tracking, and cloud-based stock management systems is making it easy to implement purchasing and distribution tasks, reducing the lead times and minimizing the possibilities of shortages or delays in trials.
In addition, the increasing interest in patient-led trial designs and decentralized clinical trials is driving demand for high-quality and adaptable supply solutions that will be able to effectively support hybrid and remote study models. Additionally, strategic alliances among pharmaceutical businesses, contract research organizations, and logistics experts are driving innovation and collaboration across the value chain, resulting in higher operational efficiency and cost savings. Furthermore, the rising trend of clinical research globalization is bringing about a budding market for supplies because of the demand for standardized products from multination studies across most geographies, which is generating economies of scale. Apart from that, more investments in clinical research units and government encouragement in the form of policies to bring about more innovation in the health care sector are further propelling the market. Lastly, increasing focus on green supply practice and sustainability is impacting the industry as stakeholders seek to reduce waste and enhance resource utilization so that clinical trial supply chains are most efficient and reliable. Overall, the intersection of these drivers—everything from tech evolution and regulation to strategic alliances and innovative study designs—is positioning the United States clinical trial supplies market for long-term success, and it is a key component of the modern clinical research picture.
Request for a sample copy of this report: https://www.imarcgroup.com/united-states-clinical-trial-supplies-market/requestsample
United States Clinical Trial Supplies Market Report Segmentation:
The United States clinical trial supplies market is segmented on the basis of services, phase, therapeutic area, and end use industry.
Services Insights:
- Product Manufacturing
- Packaging, Labeling and Storage
- Logistics and Distribution
Phase Insights:
- Phase I
- Phase II
- Phase III
- Others
Therapeutic Area Insights:
- Oncology
- Cardiovascular Diseases
- Respiratory Diseases
- Central Nervous System (CNS) and Mental Disorders
- Others
End Use Industry Insights:
- Medical Device Industry
- Biopharmaceuticals Industry
- Pharmaceuticals Industry
- Others
Regional Insights:
- Northeast
- Midwest
- South
- West
Top Players Analysis:
The report provides a detailed analysis of the competitive environment. It covers various aspects such as market structure, positioning of key players, top strategies for success, a competitive dashboard, and a company evaluation quadrant. Furthermore, the report includes comprehensive profiles of all major companies.
Ask Analyst for Customization: https://www.imarcgroup.com/request?type=report&id=20636&flag=C
Other Key Points Covered in the Report:
- COVID-19 Impact on the Market
- Porter's Five Forces Analysis
- Strategic Recommendations
- Market Dynamics
- Historical, Current and Future Market Trends
- Market Drivers and Success Factors
- SWOT Analysis
- Value Chain Analysis
- Comprehensive Mapping of the Competitive Landscape
- Top Winning Strategies
- Recent Industry News
- Key Technological Trends & Development
If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact US:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145





















Comments
0 comment