views
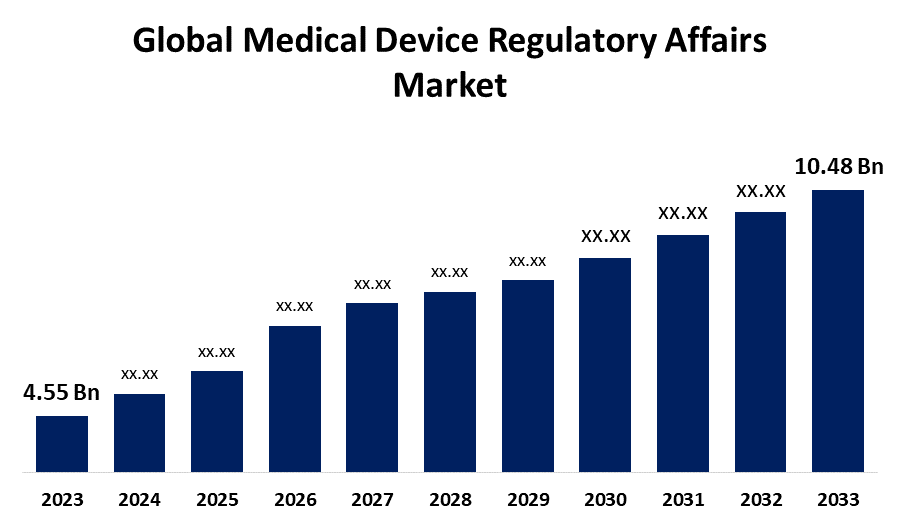
The Medical Device Regulatory Affairs Market is undergoing significant transformation, driven by the increasing complexity of global regulations and the rapid advancement of medical technologies. With the market size projected to exceed USD 10.48 billion by 2033, growing at a CAGR of 8.70% from 2023 to 2033, regulatory affairs professionals are playing a pivotal role in ensuring the safety, efficacy, and compliance of medical devices.
Download Free Sample Report: https://www.sphericalinsights.com/request-sample/5985
Why Regulatory Affairs Matter
Regulatory affairs are critical in the medical device industry, ensuring that products meet stringent safety and efficacy standards before reaching the market. From pre-market approvals to post-market surveillance, regulatory professionals navigate complex frameworks set by authorities like the FDA, EMA, and other global regulatory bodies. Their work ensures that medical devices, from simple bandages to advanced diagnostic equipment, are safe for patients and healthcare providers.
Key Drivers of Market Growth
-
Increasing Demand for Compliance:
Regulatory affairs ensure that medical devices comply with global standards, enabling companies to access international markets and avoid costly penalties. -
Rising Focus on Patient Safety:
Strict regulatory frameworks are being implemented to minimize risks associated with medical devices, ensuring patient safety and device efficacy. -
Technological Advancements:
Innovations in AI, IoT, and digital health technologies are driving the need for updated regulatory strategies to address new challenges and opportunities. -
Global Market Expansion:
Companies are expanding into emerging markets, necessitating compliance with diverse regulatory requirements across regions. -
Post-Market Surveillance:
Regulatory agencies are emphasizing post-market monitoring to ensure the ongoing safety and effectiveness of medical devices throughout their lifecycle.
Emerging Opportunities
-
Regulatory Consulting Services:
The demand for regulatory consulting is growing rapidly as companies seek expert guidance to navigate complex and evolving regulatory landscapes. -
Therapeutic Devices:
The therapeutics segment is expected to witness the fastest growth, driven by the rising prevalence of chronic diseases and advancements in medical technology. -
Digital Health and AI Integration:
The integration of AI and digital health technologies into medical devices is creating new opportunities for regulatory professionals to address unique compliance challenges. -
Emerging Markets:
Countries in Asia-Pacific and Latin America are becoming key growth areas, with increasing investments in healthcare infrastructure and regulatory frameworks.
Challenges and Restraints
Despite its growth, the market faces challenges such as:
-
High Compliance Costs: Navigating complex regulations can be time-consuming and expensive, delaying product launches.
-
Regulatory Divergence: Differences in regional regulations create hurdles for global market access.
-
Risk of Non-Compliance: Failure to meet regulatory standards can lead to product recalls, legal actions, and reputational damage.
Market Segmentation
The global medical device regulatory affairs market is segmented by:
-
Regulatory Phase: Pre-market and post-market (post-market dominates due to increased focus on surveillance).
-
Service: Regulatory consulting, product registration, legal representation, and regulatory writing.
-
Type: Therapeutics (fastest-growing segment) and diagnostics.
Verify Discount for This Report: https://www.sphericalinsights.com/request-discount/5985
Regional Insights
-
North America: Leads the market due to stringent regulatory frameworks and advanced healthcare infrastructure.
-
Europe: Strong growth driven by harmonized regulations under the EU Medical Device Regulation (MDR).
-
Asia-Pacific: Emerging as a high-growth region with increasing investments in healthcare and regulatory reforms.
Competitive Landscape
- Freiberger Compound Materials GmbH
- Nanografi Nano Technology
- Azelis Group
- American Elements
- DOWA Holdings Co., Ltd.
- AXT Inc.
- CrysTec GmbH
- Atecom Technology Co., Ltd.
- Logitech Limited
- IQE PLC
- nanoPHAB B.V.
- CMC Microsystems
- Intelligent Epitaxy Technology, Inc. (IntelliEPI)
- ALB Materials Inc.
- PlutoSemi Co., Ltd.
- Others
These companies are focusing on strategic partnerships, acquisitions, and service expansions to strengthen their market position.
For instance, in September 2022, AmerisourceBergen Corporation acquired PharmaLex Holding GmbH, enhancing its capabilities in life sciences services.
Future Outlook
The medical device regulatory affairs market is set to grow significantly, driven by the increasing complexity of regulations, technological advancements, and the need for global compliance. Companies that invest in regulatory expertise and innovative solutions will be well-positioned to capitalize on emerging opportunities.
Unlock the Full Potential of the Medical Device Regulatory Affairs Market
For a comprehensive analysis of the market, including historical data (2019-2022), forecasts (2023-2033), and detailed segmentation, explore the following resources:
Access the Full Report: https://www.sphericalinsights.com/reports/medical-device-regulatory-affairs-market
Related URL:
커팅 보드 시장 규모, 점유율, 추세 및 성장 예측
https://www.sphericalinsights.kr/reports/cutting-boards-market
공기 청정기 시장 규모, 추세, 성장 및 예측 2023~2033
https://www.sphericalinsights.kr/reports/air-purifier-market
유리 식기 시장 규모, 추세, 성장 및 예측 2023~2033
https://www.sphericalinsights.kr/reports/glass-tableware-market
렌즈 세척 제품 시장 규모, 추세 성장 및 예측 2023-2033
https://www.sphericalinsights.kr/reports/lens-cleaning-products-market
자동차 금속 시장 규모, 추세 및 2023년부터 2033년까지의 성장 예측
https://www.sphericalinsights.kr/reports/automotive-metals-market
비커 시장 규모, 추세 및 성장 예측 2023~2033
https://www.sphericalinsights.kr/reports/beaker-market
About Spherical Insights
Spherical Insights is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company's mission is to work with businesses to achieve business objectives and maintain strategic improvements.
Contact Us:
Email: sales@sphericalinsights.com
Phone: +1 303 800 4326 (US)
Follow Us: LinkedIn | Facebook | Twitter






















Comments
0 comment