views
Chemical Properties
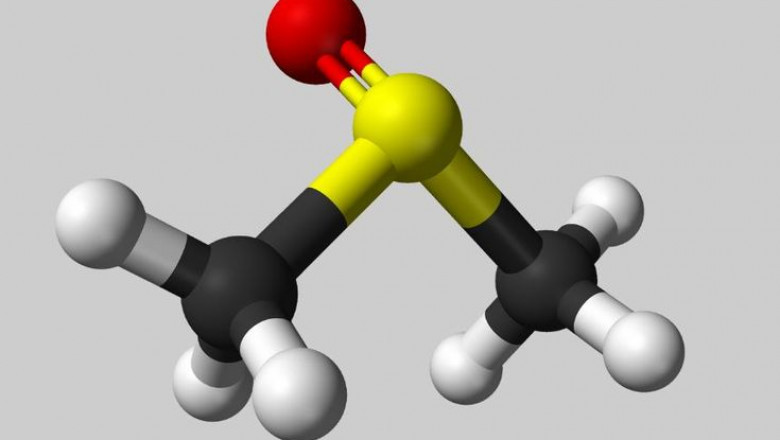
Diethyl Sulfide, also known as diethyl sulphide, is an organosulfur compound with the formula (C2H5)2S. It is a colorless liquid with an unpleasant smell often compared to rotting potatoes or cabbage. It is soluble in organic solvents but is nearly insoluble in water. Its melting point is -115°C and boiling point is 117°C. The chemical structure of it consists of two ethyl groups (C2H5) bonded to a central sulfur atom. Like other alkyl sulfides, it is stable but can undergo substitution reactions when treated with halogenating agents or oxidizing agents.
Natural Occurrence
It occurs naturally in small amounts in certain foods and plants. It is one of the sulfur compounds responsible for the characteristic smells of onions, garlic and certain other allium vegetables when chopped or crushed. Trace amounts of it can also be found in cheese, wines, beers and other alcoholic beverages. The compound is produced as a byproduct during fermentation processes by yeasts and bacteria. Diethyl Sulfide likely acts as a defense mechanism for plants against microbial pathogens or grazing animals by deterring consumption through its unpleasant smell.
Synthetic Production
While it occurs naturally, it can also be produced synthetically in the laboratory. A common method involves the reaction of sodium ethanethiolate with diethyl sulfate. Sodium ethanethiolate (C2H5SNa) is prepared by treating ethanethiol (ethyl mercaptan, C2H5SH) with sodium metal. It is then reacted with diethyl sulfate ((C2H5O)2SO4) in an SN2 reaction, where the sulfur atom is displaced. This synthetic route allows for larger scale production of purified Diethyl Sulfide.
Uses and Applications
Due to its distinct rotten smell, Diethyl Sulfide finds some niche applications where bad odors are desirable as a deterrent. It is sometimes added in small amounts to natural gas to aid in leak detection since natural gas itself is odorless. The compound is also used as a scent additive in bait and lure products for hunting and fishing. Its offensive smell acts to attract certain prey species while warning others away. In the past, it saw more widespread use as an insecticide and pest repellent for household insects before being replaced by more effective synthetic alternatives. Today, the main application of Diethyl Sulfide remains as a reagent in organic synthesis reactions.
Safety and Regulations
Like most volatile sulfur compounds, it is considered toxic if inhaled or ingested in large quantities. The American Conference of Governmental Industrial Hygienists has established a threshold limit value of 10 parts per million for occupational exposure to its vapors in workplace air. Above this level, the compound can cause eye, nose and throat irritation along with headaches. Prolonged or repeated exposure may lead to damage of the liver and kidneys as well as central nervous system effects. For these reasons, it it requires proper handling and storage according to Safety Data Sheet guidelines. No special regulations exist for its production or use beyond standard product safety labeling.
Toxicology
Research on the toxicological properties and effects of it has been fairly limited. Much of what is known about its toxicity comes from animal studies and reports of human exposure incidents. In rodents, the median lethal dose (LD50) of Diethyl Sulfide administered orally is 1710 mg/kg. At high concentrations, it primarily affects the central nervous system in mammals. Symptoms may include lethargy, ataxia, respiratory distress and possibly death from respiratory paralysis. It is readily absorbed after inhalation or ingestion and distributed throughout the body, including to fat tissues where it can accumulate. The main sites of toxicity are the kidneys and liver, where the compound undergoes oxidation and subsequent conjugation with glutathione for elimination in urine.
Metabolism and Elimination
In humans and other animals, it is rapidly absorbed, distributed and metabolized after entering the body. Its low water solubility means it can quickly permeate cell membranes and tissues. The liver represents the primary site of metabolism via oxidation reactions mediated by cytochrome P450 enzymes, such as CYP2E1. This converts it into more water-soluble metabolites including diethyl sulfone, diethyl sulfoxide and their corresponding glucuronide and glutathione conjugates. These metabolites can then be eliminated renally within 24-48 hours, mainly through urine but also a small amount in exhaled air. Due to its volatility, eliminating unchanged Diethyl Sulfide by exhalation also occurs to some degree. Overall, the metabolic and elimination pathways allow most ingested or inhaled it to be cleared quickly from the body.
For Better Understanding, choose preferred language-
About Author-
Money Singh is a seasoned content writer with over four years of experience in the market research sector. Known for her strong SEO background, she skillfully blends SEO strategies with insightful content. Her expertise spans various industries, including food and beverages, biotechnology, chemical and materials, defense and aerospace, consumer goods, etc. (https://www.linkedin.com/in/money-singh-590844163)





















Comments
0 comment