views
Report Overview
The Global Atopic Dermatitis Drugs Market size is expected to be worth around US$ 24.5 Billion by 2033, from US$ 10.5 Billion in 2023, growing at a CAGR of 9.1% during the forecast period from 2024 to 2033.
The Atopic Dermatitis Drugs Market is entering a breakthrough phase in 2025 with the introduction of nanotechnology-based formulations and dual-action biologics. These new therapies are designed to improve skin penetration and target multiple inflammatory pathways simultaneously, reducing flare-ups and minimizing side effects. Companies are investing heavily in next-gen delivery systems, including lipid nanoparticles and polymeric gels that enhance drug absorption.
Biopharma startups are also gaining ground by offering subscription-based therapy models, improving accessibility and adherence. Regulatory bodies are accelerating approvals for drugs with multifunctional mechanisms, while payers are expanding support for high-efficacy treatments. This evolution reflects a broader healthcare shift toward targeted, sustained-release dermatological care, positioning the atopic dermatitis drug market to surpass previous growth forecasts, especially in North America and Asia-Pacific.
Click here for more information: https://market.us/report/atopic-dermatitis-drugs-market/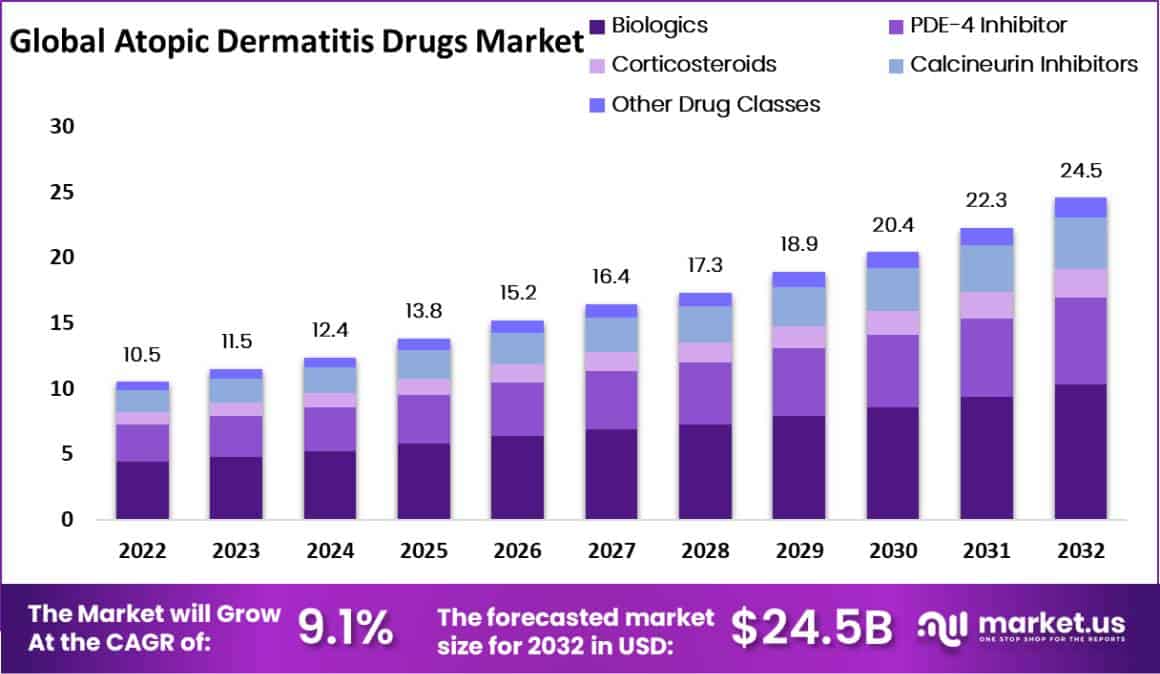
Key Market Segments
Based on Drug Class
-
- Biologics
- PDE-4 Inhibitor
- Corticosteroids
- Calcineurin Inhibitors
- Other Drug Classes
Based on the Route of Administration
-
- Injectable
- Topical
- Oral
Based on Distribution Channel
-
- Hospital Pharmacies
- Online Pharmacies
- Retail Pharmacies
Market Key Players
-
- Sanofi SA
- Pfizer Inc.
- AbbVie Inc.
- Eli Lilly and Company
- LEO Pharma Inc.
- Novartis AG
- Otsuka Pharmaceutical Co., Ltd.
- Regeneron Pharmaceuticals Inc.
- Other Key Players
Get a Sample Copy of the Report to Know More: https://market.us/report/atopic-dermatitis-drugs-market/request-sample/
Emerging Trends
One major trend in 2025 is the use of nanocarrier drug delivery systems, which significantly increase topical drug efficacy and reduce irritation. These nanocarriers enable controlled release, making them ideal for chronic conditions like AD. Another growing trend is dual-pathway biologics, which simultaneously inhibit IL‑4 and IL‑31 for more comprehensive symptom relief.
Pharma companies are also introducing value-based pricing models, aligning costs with outcomes. This is encouraging wider adoption among healthcare systems in both developed and emerging markets.
Use Cases
Hospitals are adopting dual-action biologics for patients who previously showed limited response to single-pathway drugs, improving skin clearance scores significantly. Nanoparticle-infused creams are being used in clinical trials for hard-to-treat areas like eyelids and hands. Pediatric clinics report better compliance with once-weekly spray formulations over daily ointments.
In teledermatology programs, patients upload photos via apps integrated with their prescription plans, enabling physicians to tailor dosages without requiring physical visits. These approaches reflect a move toward personalized and convenient care delivery.
Contact us on
Market.us (Powered By Prudour Pvt. Ltd.)
Email: inquiry@market.us
Address: 420 Lexington Avenue, Suite 300,
New York City, NY 10170, United States
Tel: +1 718 618 4351






















Comments
0 comment