views
In recent years, the management of atopic dermatitis (AD) has taken a significant leap forward, thanks to innovative treatments that target the underlying mechanisms of the disease. One such breakthrough is OPZELURA (ruxolitinib) cream—a topical formulation that is reshaping how healthcare providers and patients approach this chronic skin condition. In this article, we delve into the transformative potential of OPZELURA, exploring its clinical efficacy, mechanism of action, and future prospects.
For more in-depth insights on OPZELURA’s development and future potential, download the full report @ OPZELURA Market Report
OPZELURA for Atopic Dermatitis: A Breakthrough in Topical Treatment
At the forefront of dermatological advancements, OPZELURA represents a pivotal shift from conventional therapies. Unlike traditional treatments that often rely on steroids or systemic immunosuppressants, OPZELURA harnesses a targeted approach to reduce inflammation at its source. The formulation, with its active ingredient ruxolitinib, is designed specifically for patients who need a non-steroidal option to manage their mild to moderate atopic dermatitis. This breakthrough has not only provided clinicians with a novel treatment avenue but also led to robust market acceptance—evidenced by impressive OPZELURA sales figures that continue to grow as more patients and practitioners embrace its benefits.
Understanding Atopic Dermatitis
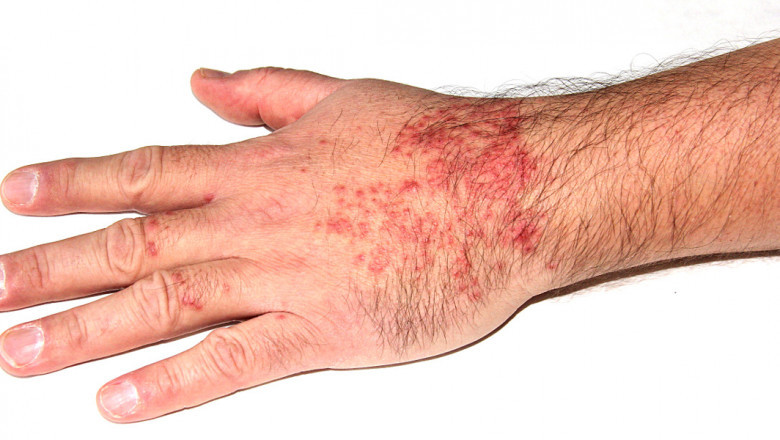
Atopic dermatitis, commonly known as eczema, is a multifactorial, chronic inflammatory skin condition that affects millions worldwide. Characterized by dry, scaly skin, intense itching, red inflamed patches, and even skin cracking or weeping, AD can severely impact an individual’s quality of life. Its etiology is complex, involving a combination of genetic predispositions, environmental triggers, and dysregulated immune responses. Traditionally, treatment modalities have included emollients, corticosteroids, and calcineurin inhibitors. However, long-term use of some of these therapies—particularly corticosteroids—carries risks such as skin thinning and hormonal imbalances, prompting the need for safer and more effective alternatives.
For more detailed insights and the latest updates on OPZELURA, visit the OPZELURA Market update
Introduction to OPZELURA (Ruxolitinib)
OPZELURA is a revolutionary topical cream formulated with ruxolitinib, a potent Janus kinase (JAK) inhibitor. Developed by Incyte Corporation, this product received U.S. Food and Drug Administration (FDA) approval in September 2021 for treating mild to moderate atopic dermatitis in patients aged 12 and older who are not immunocompromised. This OPZELURA approval milestone has been significant not only in validating its clinical promise but also in setting a new standard for topical treatments in dermatology. As the OPZELURA active ingredient, ruxolitinib directly targets inflammatory pathways, offering patients a viable alternative when conventional therapies fall short.
Mechanism of Action (MOA)
The innovation behind OPZELURA lies in its precise mechanism of action. By inhibiting the JAK1 and JAK2 pathways, OPZELURA’s Mechanism of Action interferes with the JAK-STAT signaling cascade—a critical pathway involved in mediating immune responses and inflammatory processes. The blockade of these pathways leads to a marked reduction in inflammatory cytokine activity, thereby diminishing skin inflammation, reducing itch severity, and promoting improved skin barrier function. This targeted approach is a key differentiator from traditional therapies and is reflected in the favorable outcomes observed in OPZELURA Clinical Trials, where rapid symptom relief was noted.
For further insights and detailed research on this breakthrough treatment, visit OPZELURA insights
Clinical Efficacy of OPZELURA
Clinical evidence has been pivotal in establishing the role of OPZELURA as a transformative treatment for atopic dermatitis. Multiple clinical studies have demonstrated its efficacy in alleviating the symptoms of AD, showcasing significant improvements in skin clarity and patient-reported outcomes. These OPZELURA Clinical Trials have highlighted the cream’s ability to deliver rapid relief from itching and inflammation, often within the first 24 to 48 hours of application. Moreover, the favorable safety profile—with minimal systemic absorption—underscores its potential for long-term use without the side effects commonly associated with systemic therapies.
Phase 3 TRuE-AD Trials
The Phase 3 TRuE-AD1 and TRuE-AD2 trials were instrumental in confirming the efficacy of OPZELURA. These pivotal studies involved over 1,200 patients with mild to moderate AD and provided robust data that propelled the treatment into the spotlight. A significant proportion of trial participants achieved clear or nearly clear skin as measured by the Investigator’s Global Assessment (IGA) score. In addition, improvements in the eczema area and severity index (EASI) scores were noted, alongside a rapid reduction in itch severity. The success of these trials has been a major contributing factor to the impressive OPZELURA sales seen in the market today, reflecting a high level of confidence among dermatologists.
For additional insights on OPZELURA’s transformative potential, please download the full OPZELURA report
Real-World Effectiveness
Beyond controlled clinical environments, real-world data reinforces the efficacy and tolerability of OPZELURA. Patients using the cream have reported sustained relief from the hallmark symptoms of atopic dermatitis, particularly the debilitating itch that disrupts sleep and daily activities. This real-world effectiveness has contributed to steadily increasing OPZELURA sales, as more patients experience improved quality of life with fewer adverse effects. Healthcare providers have recognized the clinical benefits of OPZELURA, which further bolsters its reputation as a reliable and innovative treatment option.
Safety Profile and Side Effects
While OPZELURA is celebrated for its efficacy, its safety profile is equally noteworthy. The cream is generally well tolerated, with most patients experiencing only minor side effects. Reported issues include transient application site reactions—such as mild redness, burning, or stinging—along with occasional headaches or nasopharyngitis symptoms. Importantly, because of the minimal systemic absorption of ruxolitinib when applied topically, the risk of systemic side effects is considerably lower compared to oral JAK inhibitors. This balance between efficacy and safety is a critical aspect of OPZELURA’s appeal, particularly for long-term management of atopic dermatitis.
Comparisons with Other Treatments
When compared to traditional therapies, OPZELURA offers several compelling advantages. Its targeted, non-steroidal formulation sets it apart from both corticosteroids and calcineurin inhibitors. Unlike topical corticosteroids, OPZELURA does not lead to skin thinning or hormonal imbalances. Similarly, while calcineurin inhibitors can cause a burning sensation upon application, patients using OPZELURA generally experience a smoother and more comfortable treatment experience. Furthermore, for patients requiring treatment beyond localized application, systemic therapies such as dupilumab or baricitinib are often reserved for more severe cases. In contrast, OPZELURA fills a crucial gap by providing effective relief for localized AD without the systemic side effects.
OPZELURA vs. Topical Corticosteroids
Topical corticosteroids have long been the standard of care for managing atopic dermatitis. However, their prolonged use can result in adverse effects such as skin atrophy and suppression of the hypothalamic-pituitary-adrenal (HPA) axis. OPZELURA, by contrast, offers a similar level of efficacy in reducing inflammation and itch without these complications. Its non-steroidal nature makes it a safer long-term option for patients who require continuous management of their symptoms.
OPZELURA vs. Calcineurin Inhibitors (Tacrolimus, Pimecrolimus)
Calcineurin inhibitors, including tacrolimus and pimecrolimus, provide an alternative to steroids but are not without their drawbacks. Many patients report a burning sensation when these products are applied, and their usage in younger populations often occurs off-label. OPZELURA’s formulation is designed for patient comfort, and its rapid onset of action—evident in the clinical trials—positions it as a more appealing option for those seeking effective symptom control with minimal discomfort.
OPZELURA vs. Systemic Therapies (Dupilumab, Baricitinib)
For moderate to severe atopic dermatitis, systemic therapies like dupilumab or baricitinib are typically considered. However, these treatments involve systemic administration and are often accompanied by a higher risk of side effects. OPZELURA’s topical delivery allows for localized treatment, thereby reducing systemic exposure and associated risks. This makes OPZELURA particularly advantageous for patients with localized disease manifestations who are looking for an effective yet safe alternative.
For those looking to explore more about this breakthrough treatment, download the full OPZELURA Insights Report
Patient Considerations
When considering treatment options for atopic dermatitis, patient-specific factors play a crucial role. OPZELURA offers a tailored solution for individuals who have not achieved adequate relief from conventional therapies. Its rapid action in alleviating itch and inflammation has been linked to better patient adherence and overall satisfaction. Furthermore, the targeted mechanism of OPZELURA reduces the risk of adverse effects commonly seen with long-term steroid use, making it a favorable option for both younger patients and those with chronic conditions.
Who Can Use OPZELURA?
OPZELURA is particularly recommended for patients with mild to moderate atopic dermatitis who require an alternative to traditional topical corticosteroids. It is ideally suited for individuals who experience frequent flare-ups and are in need of a long-term treatment plan that minimizes the risk of steroid-related complications. Approved for patients aged 12 and older, OPZELURA provides a safe and effective solution for a broad demographic, ensuring that many can benefit from its innovative approach.
Who Should Avoid OPZELURA?
While OPZELURA is a promising treatment, it may not be suitable for everyone. Patients with active infections or those with a history of serious cardiovascular or thromboembolic conditions should exercise caution and consult their healthcare provider before using the cream. Additionally, use in pregnant or breastfeeding women should only occur under strict medical supervision, ensuring that the benefits outweigh any potential risks.
Future Perspectives
The advent of OPZELURA marks not only a milestone in the treatment of atopic dermatitis but also opens new avenues for addressing other inflammatory skin disorders. Ongoing research is exploring its efficacy in conditions such as vitiligo, psoriasis, and alopecia areata. The continued study of OPZELURA’s Mechanism of Action is expected to optimize its formulation and broaden its clinical applications. As the scientific community gathers more data, future iterations of JAK inhibitors for topical use are likely to further refine the balance between safety and efficacy, potentially revolutionizing dermatological care on a global scale.
Conclusion
OPZELURA (ruxolitinib) cream has emerged as a transformative treatment for atopic dermatitis, offering a much-needed non-steroidal alternative with a rapid onset of action and an excellent safety profile. Its targeted mechanism—focusing on inhibiting key inflammatory pathways—has not only delivered promising results in rigorous clinical trials but also translated into real-world effectiveness that significantly improves patients’ quality of life. With the dual advantage of reducing the risks associated with long-term steroid use and providing rapid relief from debilitating symptoms, OPZELURA is setting new benchmarks in the field of dermatology. As ongoing research continues to unveil additional benefits and potential applications, OPZELURA Approvals and OPZELURA Clinical Trials remain at the heart of this innovation, driving its widespread acceptance and robust OPZELURA sales worldwide.
Related Reports
-
Atopic Dermatitis Market Insight, Epidemiology and Market Forecast
-
Radiation Dermatitis Market Insight, Epidemiology and Market Forecast
-
Seborrhoeic Dermatitis - Market Insight, Epidemiology and Market Forecast
About DelveInsight
DelveInsight is a leading business Healthcare consultancy and market research firm specializing in life sciences. It assists pharmaceutical companies by offering comprehensive, end-to-end solutions to improve their performance. Access all our healthcare and pharmaceutical market Competitive Intelligence Solutions.





















Comments
0 comment