views
The hidradenitis suppurativa (HS) market is undergoing a profound transformation, propelled by rapid advances in therapeutic research, evolving patterns of disease prevalence, and heightened recognition of unmet clinical needs worldwide. Hidradenitis suppurativa, a chronic, inflammatory skin disorder marked by recurring, painful nodules and abscesses in intertriginous regions, profoundly affects patients’ quality of life due to its cyclical pattern of inflammation, healing, and scarring. Unlike many other dermatological diseases, HS poses unique market challenges owing to its chronic progression, frequent delays in diagnosis, and the historic lack of effective therapies. This article explores the current market dynamics, epidemiological trends, recent clinical and therapeutic developments, competitive landscape, as well as the crucial drivers and market barriers defining the future outlook for hidradenitis suppurativa management.
For insights into the emerging trends and market dynamics shaping the future of Hidradenitis Suppurativa care, explore our in-depth analysis of Hidradenitis Suppurativa treatment market insights.
Hidradenitis Suppurativa Market Overview
The global hidradenitis suppurativa market is predicted to experience substantial growth over the next decade, with industry analysts anticipating a robust compound annual growth rate (CAGR). This accelerating expansion is primarily underpinned by increased disease awareness, advancements in diagnostic methodologies, and the advent of innovative biologic therapies that target underlying inflammatory pathways. Historically, treatment for HS revolved around antibiotics and surgical interventions, which delivered inconsistent results. However, the endorsement and uptake of biologics, most notably adalimumab and the more recently approved secukinumab, have revolutionized the therapeutic approach. The entry of bimekizumab in the European market and its ongoing regulatory review in the United States further reflect this paradigm shift. These therapies not only enhance clinical outcomes but also signify robust industry investment in research and development, highlighting the growing commercial and clinical interest in addressing the significant unmet need within the HS patient community.
Epidemiology and Clinical Burden
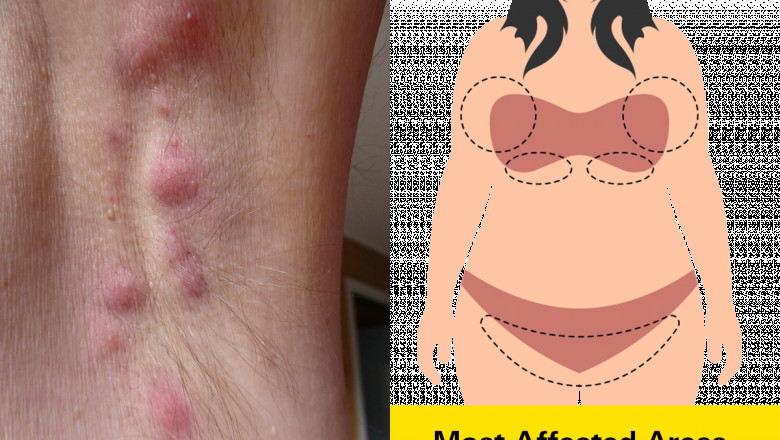
Epidemiological research into hidradenitis suppurativa reveals noteworthy regional variability in disease prevalence. Western Europe, Scandinavia, the United States, and Australia have generated the bulk of prevalence data, with Europe and the US reporting relatively higher rates compared to other regions. A notable gender disparity exists, with females predominantly affected in North America and Europe, contrasted by a higher male prevalence in certain Asian populations such as South Korea. HS most frequently appears in late adolescence or early adulthood, with incidence rates peaking among young adults. Interestingly, the prevalence seems to decline with age, suggesting age-related biological or environmental factors influence disease expression. Despite improved awareness in the West, significant gaps remain in the epidemiological mapping of HS in non-Western nations, underscoring a key opportunity for future research and for companies operating in global markets to better define the full extent of the disease burden.
For further insights and detailed research on Hidradenitis Suppurativa epidemiology, visit the Hidradenitis Suppurativa patient pool.
Recent Therapeutic Developments and Market Drivers
Key drivers propelling the hidradenitis suppurativa market include the growing recognition of HS as a systemic, inflammatory comorbidity and the validation of immunological pathways as viable therapeutic targets through successful biologic treatments. The development and approval of anti-TNF therapies, such as adalimumab, have paved the way for further biologics, creating a competitive environment where pharmaceutical companies actively invest in newer agents like anti-IL-17 therapies (e.g., secukinumab and bimekizumab). Enhanced disease awareness among both clinicians and patients is contributing to earlier, more accurate diagnoses, although significant diagnostic delays persist. Moreover, the severe impact of HS on quality of life, manifesting as physical discomfort and psychosocial distress, amplifies demand for comprehensive and more effective treatments. A growing trend towards personalized medicine—tailoring therapy based on individual inflammatory and clinical profiles—holds promise for better patient outcomes and is increasingly shaping the research and commercial strategies in this field.
Market Barriers and Unmet Needs
Despite encouraging advancements, the hidradenitis suppurativa market continues to grapple with daunting challenges. Diagnostic delays remain widespread, with many patients enduring symptoms for years before receiving a definitive diagnosis. A considerable proportion of patients experience inadequate symptom control or progressive disease, even when treated with the most advanced therapies. The multifactorial, relapsing-remitting nature of HS necessitates an integrated, multidisciplinary management approach that goes beyond skin symptoms, addressing systemic comorbidities like metabolic syndrome and psychological distress. The lack of targeted, approved therapies for mild HS—a segment largely overlooked by current biologic approvals—represents a substantial unmet need. Additionally, the complexity and heterogeneity of HS pathophysiology demand further research and biomarker validation to support better treatment selection and clinical trial design.
Competitive Landscape and Pipeline Innovation
The HS therapeutic pipeline is notably rich and diverse, reflecting the clinical and commercial emphasis on unraveling the underlying immunopathology. Alongside established biologics like adalimumab and secukinumab, pipeline agents such as bimekizumab (dual-acting IL-17A and IL-17F inhibitor) and sonelokimab (a nanobody with increased tissue penetration) are being actively investigated. JAK inhibitors, particularly povorcitinib, are poised to become the first oral options in the HS segment, potentially enhancing patient convenience and adherence. Several other investigational compounds are targeting novel pathways, including IL-36, IL-1, and chemokine (CXCR1/CXCR2) inhibition. Topical innovations like ruxolitinib cream are particularly promising for patients with mild or early-stage disease—a population currently underserved by the available systemic therapies. This dynamic competitive landscape, buoyed by robust clinical trial activity, is expected to trigger further market expansion and product differentiation in the coming years.
For detailed insights on emerging therapies and trends within the Hidradenitis Suppurativa treatment market, download the full report.
Future Directions and Conclusion
The hidradenitis suppurativa market is entering a pivotal era, balancing significant therapeutic optimism with ongoing clinical challenges. While the arrival of novel biologics and a vibrant pipeline underscore real progress, achieving consistent long-term disease control and quality-of-life improvement remains elusive for many patients. Looking ahead, the integration of biomarkers for treatment stratification, rapid advances in personalized medicine, and adoption of multidisciplinary care models that address both physical and psychosocial aspects are poised to redefine HS management. As pharmaceutical companies intensify research efforts and regulators foster accelerated pathways for innovation, the next decade promises to deliver transformative changes in the therapeutic landscape. Ultimately, the ongoing evolution in HS market dynamics holds the potential to bring new hope, improved outcomes, and a higher standard of care to the global HS patient community.
For further insights and detailed updates on this evolving field, visit our comprehensive insights and expert analysis.
Read More
About DelveInsight
DelveInsight is a leading business Healthcare consultancy and market research firm specializing in life sciences. It assists pharmaceutical companies by offering comprehensive, end-to-end solutions to improve their performance. Access all our healthcare and pharmaceutical market Competitive Intelligence Solutions.





















Comments
0 comment