views
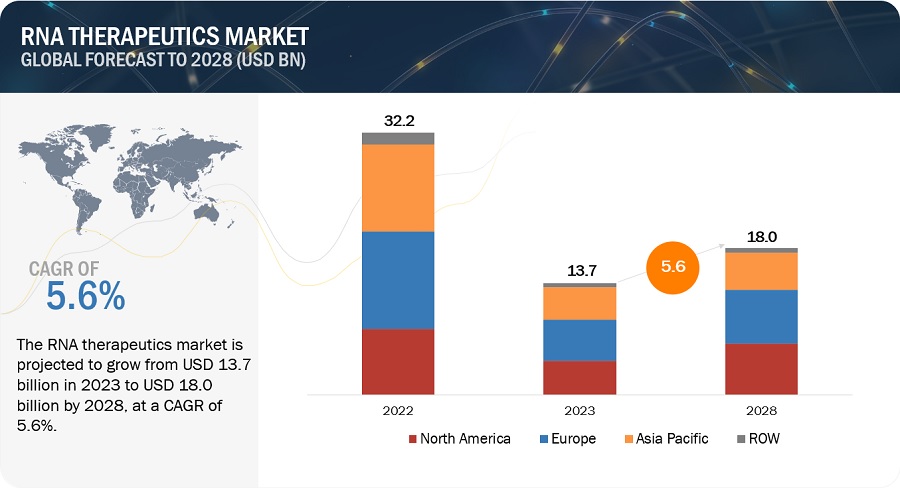
The size of global RNA therapeutics market in terms of revenue was estimated to be worth $13.7 billion in 2023 and is poised to reach $18.0 billion by 2028, growing at a CAGR of 5.6% from 2023 to 2028. The research study consists of industry trends, pricing analysis, patent analysis, conference and webinar materials, key stakeholders, and buying behaviour in the market.
Growth in this market is largely driven by factors such as the increasing number of partnerships and collaborations among market players and RNA technology manufacturers, expanding modalities for RNA therapeutics, and the rising number of emergency use authorizations and approvals for COVID-19 booster vaccines. On the other hand, the discontinuation/recalls of RNA therapeutic products is expected to hinder market growth.
Download an Illustrative overview: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=235963408
RNA therapeutics, which include messenger RNA (mRNA) therapies, small interfering RNA (siRNA) therapies, and antisense oligonucleotides (ASOs), have gained significant attention due to their ability to target diseases at the genetic level. These therapies are becoming a critical part of personalized medicine, offering new treatment options for conditions that have been challenging to address with traditional approaches.
Key factors contributing to the market's expansion include:
-
Technological Advancements: Breakthroughs in RNA delivery systems and improved stability of RNA molecules are enabling more effective and targeted therapies.
-
Rising Prevalence of Chronic Diseases: The increasing incidence of conditions such as cancer, cardiovascular diseases, and genetic disorders is driving the demand for innovative treatment options.
-
Growing Biotech Investments: Significant investments by pharmaceutical companies and biotech firms are accelerating the development of RNA-based therapies, leading to a robust pipeline of new drugs.
-
Regulatory Support: Favorable regulatory frameworks and expedited approval processes for RNA-based treatments are further supporting market growth.
The RNA Therapeutics market is expected to see a strong compound annual growth rate (CAGR) over the forecast period, driven by these factors and ongoing research and development efforts. Leading players in the market are focusing on strategic collaborations, partnerships, and acquisitions to enhance their product portfolios and expand their market presence.
Request Sample Pages: https://www.marketsandmarkets.com/requestsampleNew.asp?id=235963408
RNA Therapeutics Market Dynamics:
Drivers:
1. Increasing partnerships and collaborations among market players and RNA technology manufacturers
Restraints:
1. Discontinuation or recalls of RNA therapeutic products
Opportunities:
1. Higher progress in the development of RNA aptamer-based therapeutics
Challenge:
1. Rapid degradation by ubiquitous RNases in environment and tissues with strong immunogenicity of exogenous RNA
Key Market Players:
The prominent players in the global RNA therapeutics market include Moderna, Inc. (US), Alnylam Pharmaceuticals, Inc. (US), Pfizer Inc. (US), Novartis AG (Switzerland), Ionis Pharmaceuticals, Inc. (US), Sarepta Therapeutics, Inc. (US), Sanofi (France), Arrowhead Pharmaceuticals, Inc. (US), BioNtech SE (Germany), Orna Therapeutics (US), CRISPR Therapeutics (Switzerland), Silence Therapeutics (UK), Astellas Pharma Inc. (Japan), CureVac SE (Germany), Sirnaomics (US), Arcturus Therapeutics Inc. (US) and Arbutus Biopharma (US).
Get 10% Free Customization on this Report: https://www.marketsandmarkets.com/requestCustomizationNew.asp?id=235963408
RNA Therapeutics Market Advantages:
- Precision Medicine: RNA-based therapies can be tailored to target specific genetic mutations or disease pathways, allowing for personalized treatments that are more effective and have fewer side effects.
- Speed of Development: The rapid development of RNA-based vaccines during the COVID-19 pandemic demonstrated the agility of this technology, which can potentially accelerate the response to emerging diseases and medical challenges.
- Versatility: RNA can be engineered to address a wide range of diseases, including genetic disorders, cancer, infectious diseases, and rare diseases, making it a versatile platform for therapeutic development.
- Reduced Off-Target Effects: Compared to traditional small molecule drugs, RNA therapies can have a higher degree of specificity, minimizing off-target effects on healthy cells and tissues.
- Lower Manufacturing Costs: Advances in RNA synthesis and production techniques are driving down manufacturing costs, potentially making RNA therapies more accessible and affordable.
- Targeting Undruggable Proteins: RNA therapeutics can target proteins that were previously considered "undruggable" with small molecules, expanding the range of potential drug targets.
- Potential for Gene Editing: RNA-based therapies, such as CRISPR-Cas9, hold promise for precise gene editing and the treatment of genetic diseases at their root cause.
- Immunomodulation: RNA molecules can be designed to stimulate or inhibit immune responses, making them valuable tools in the treatment of autoimmune diseases and for enhancing cancer immunotherapies.
- Reduced Development Risk: The ability to rapidly iterate and optimize RNA therapies in preclinical and clinical stages can reduce the risks associated with drug development.
- Therapeutic Innovation: RNA therapeutics represent a novel class of drugs that can complement existing treatment modalities, offering innovative solutions to unmet medical needs.
These advantages highlight the transformative potential of RNA therapeutics in advancing healthcare and improving patient outcomes in the near future.
Recent Developments:
- In February 2023, Moderna, Inc. received authorization from Health Canada for its COVID-19 booster vaccine, mRNA-1273.214 (SpikevaxBivalent Original/Omicron). This vaccine is designed for immunization against COVID-19 in children and adolescents between 6 to 17 years.
- In January 2023, the US FDA granted Breakthrough Therapy Designation for Moderna's investigational mRNA vaccine candidate—mRNA-1345. This vaccine was developed to prevent RSV-associated lower respiratory tract disease (RSV-LRTD) in adults aged 60 years or older.
- In September 2022, Alnylam Pharmaceuticals, Inc. received marketing authorization from the European Commission for its RNAi therapy—AMVUTTRA. This treatment is developed for adult patients with stage 1 or stage 2 polyneuropathy, which is hereditary transthyretin-mediated (hATTR).






















Comments
0 comment