views
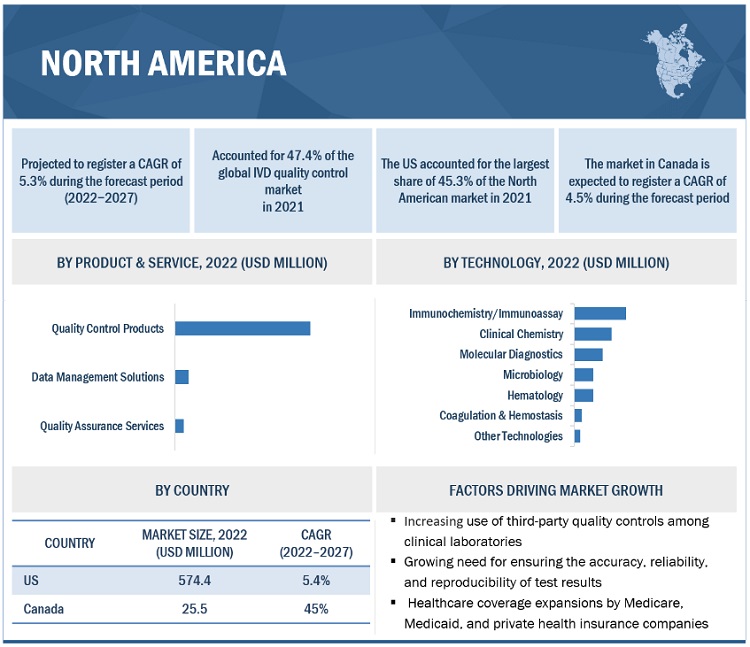
IVD Quality Control Market in terms of revenue was estimated to be worth $1.3 billion in 2022 and is poised to reach $1.6 Billion by 2027, growing at a CAGR of 5.4% from 2022 to 2027 according to a new report by MarketsandMarkets™. The growth of the IVD quality control market is driven by the rising number of accredited clinical laboratories, rising geriatric population and subsequent growth in the prevalence of chronic and infectious diseases and increasing adoption of third-party quality controls. The rising focus on multi-analyte controls and Increasing investments from government bodies and private players in healthcare sectors in emerging economies is also expected to offer significant growth opportunities for the market in the coming years. Lack of regulations for clinical laboratory accreditation in several emerging countries could be the challenges faced by the market in upcoming years.
Download an Illustrative overview: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=198032582
The latest report on the In Vitro Diagnostics (IVD) Quality Control Market provides an in-depth analysis of the current industry landscape, revealing significant growth drivers, emerging trends, and future market projections. As the demand for accurate and reliable diagnostics increases, the market is poised for substantial growth, driven by advancements in technology, rising incidences of chronic diseases, and the growing importance of quality control in clinical laboratories.
Key Growth Drivers:
- Technological Advancements: Innovations in diagnostic tools and automation are enhancing the accuracy and efficiency of in vitro diagnostics, leading to a higher demand for quality control products to ensure consistent performance.
- Rising Prevalence of Chronic Diseases: The increasing global burden of chronic diseases such as cancer, diabetes, and cardiovascular conditions is driving the need for reliable diagnostic tests, further emphasizing the importance of quality control measures.
- Regulatory Stringency: Stricter regulations and guidelines from healthcare authorities across the globe are mandating the use of robust quality control practices, fueling the growth of the IVD quality control market.
- Expansion of Point-of-Care Testing: The shift towards decentralized testing and the growing adoption of point-of-care diagnostics are creating new opportunities for quality control products to ensure the accuracy and reliability of these tests.
Emerging Trends:
- Integration of Artificial Intelligence (AI): AI and machine learning are being increasingly incorporated into diagnostic tools, requiring more sophisticated quality control systems to validate the accuracy of AI-driven results.
- Focus on Personalized Medicine: The movement towards personalized healthcare is driving the demand for customized quality control solutions that cater to specific diagnostic tests.
- Sustainability Initiatives: Companies are prioritizing environmentally friendly practices, leading to the development of sustainable quality control products that reduce waste and energy consumption.
Market Outlook:
The IVD quality control market is expected to witness robust growth over the next few years. Key players in the industry are focusing on strategic partnerships, product innovations, and expanding their geographic presence to capitalize on emerging opportunities.
Request for FREE Sample Pages: https://www.marketsandmarkets.com/requestsampleNew.asp?id=198032582
IVD Quality Control Market Dynamics:
Drivers:
- Increasing number of accredited clinical laboratories
- Growing adoption of third-party quality controls
- Rising demand for external quality assessment support
- Rising geriatric population and subsequent growth in the prevalence of chronic and infectious diseases
- Increasing adoption of POC instruments in developed regions
Restraints:
- Additional costs and budget constraints in hospitals and laboratories
- Unfavorable reimbursement scenario for IVD tests
Opportunities:
- Rising demand for multi-analyte controls
Challenges:
- Stringent product approval process
- Lack of regulations for clinical laboratory accreditation in several emerging countries
Key Market Players:
Some of the key players in the market include Bio-Rad Laboratories, Inc. (US), Randox Laboratories Ltd. (UK), Thermo Fisher Scientific, Inc. (US), LGC Limited (UK), and Abbott Laboratories (US). Other prominent payers in the market include Roche Diagnostics (Switzerland), Siemens Healthineers (Germany), Danaher Corporation (US), Fortress Diagnostics (UK), SERO AS (US), Sysmex Corporation (Japan), Ortho-Clinical Diagnostics (US), Helena Laboratories Corporation (US), Quidel Corporation (US), Sun Diagnostics, LLC (US), Seegene Inc. (South Korea), ZeptoMetrix Corporation (US), Qnostics (UK), Bio-Techne Corporation (US), Microbiologics (US), Microbix Biosystems (Canada), Streck, Inc. (US), Alpha-Tec Systems (US), Maine Molecular Quality Controls, Inc. (US), and Grifols, S.A. (Spain).
Get 10% Free Customization on this Report: https://www.marketsandmarkets.com/requestCustomizationNew.asp?id=198032582
About MarketsandMarkets™:
MarketsandMarkets™ is a blue ocean alternative in growth consulting and program management, leveraging a man-machine offering to drive supernormal growth for progressive organizations in the B2B space. We have the widest lens on emerging technologies, making us proficient in co-creating supernormal growth for clients.
The B2B economy is witnessing the emergence of $25 trillion of new revenue streams that are substituting existing revenue streams in this decade alone. We work with clients on growth programs, helping them monetize this $25 trillion opportunity through our service lines – TAM Expansion, Go-to-Market (GTM) Strategy to Execution, Market Share Gain, Account Enablement, and Thought Leadership Marketing.
Built on the ‘GIVE Growth’ principle, we work with several Forbes Global 2000 B2B companies – helping them stay relevant in a disruptive ecosystem. Our insights and strategies are molded by our industry experts, cutting-edge AI-powered Market Intelligence Cloud, and years of research. The KnowledgeStore™ (our Market Intelligence Cloud) integrates our research, facilitates an analysis of interconnections through a set of applications, helping clients look at the entire ecosystem and understand the revenue shifts happening in their industry.






















Comments
0 comment