views
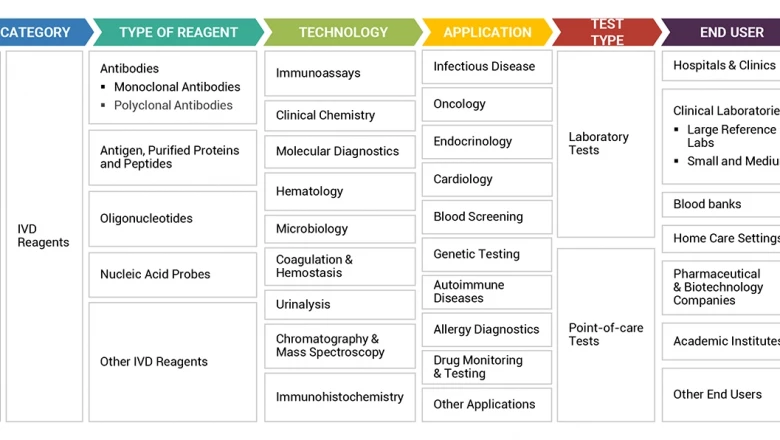
In Vitro Diagnostic (IVD) Reagents Market is Segmented by Type (Immune Diagnosis, Clinical and Biochemical, Molecular Diagnosis, POCT), by Application (Laboratory, Hospital).
The Global In Vitro Diagnostic (IVD) Reagents revenue was USD 38370 Million in 2022 and is forecast to a readjusted size of USD 55320 Million by 2029 with a CAGR of 5.3% during the forecast period (2023-2029).
Major Factors Driving the Growth of IVD Reagents Market:
The IVD reagents market is thriving, driven by the rising prevalence of chronic and infectious diseases, growing focus on preventive healthcare, and advancements in molecular diagnostics. Key end-users, including hospitals and POCT settings, ensure sustained demand for high-quality reagents. Regional markets, led by North America and Asia-Pacific, reflect diverse healthcare needs and evolving diagnostic practices. As manufacturers innovate with specialized reagents and regulatory frameworks support quality assurance, the global IVD reagents market is poised for sustained growth, meeting the demands of a dynamic healthcare landscape.
Download PDF Brochure: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=131261429
DRIVER: Gradual shift toward point-of-care testing and automated analyzers
The amount of testing conducted outside traditional laboratories is expected to grow in the coming years, driven by the increasing demand for delivering care closer to patients. The range of tests available through point-of-care (POC) testing reagents has significantly expanded recently. For example, immunoassay-based POC testing analyzers offer high sensitivity while ensuring patient compliance. Additionally, these systems help reduce the time needed to diagnose and manage various diseases, such as HIV, malaria, dengue, tuberculosis, and hepatitis C. As a result of these benefits, POC testing products are becoming more widely used to support informed clinical decisions and improve healthcare outcomes.
RESTRAINT: Stringent regulatory requirements
Regulatory and legal requirements applied to IVD (including infectious disease diagnostics) in the US and European countries are becoming more stringent. In the US, IVD products are defined under 21 CFR 809 and regulated under guidelines similar to medical reagents. The FDA released new FDA guidance documents. Under US federal regulations, device manufacturers must submit a 510(k) application for further modifications to a device. New applications may require software updates and installations in an existing device or any other changes made to these reagents.
The EU has moved from Directive 98/79/EC and several earlier directives to the In Vitro Diagnostic Regulation (IVDR) (Regulation (EU) 2017/746), and products must be (Conformité Européenne) CE-marked to be legally marketed in the EU according to the new regulatory framework. The IVDR had a transition period which ended on May 26, 2022, and it went into force on May 26, 2017. The time frame required for regulatory approval for some IVD is uncertain, and investments made in R&D may go in vain if the regulatory authority denies approval.
OPPORTUNITY: Rising significance of companion diagnostics
Companion diagnostics include tests or assays intended to assist healthcare providers in making treatment decisions for patients based on the best response to therapy. The co-development of companion diagnostics with therapeutic products can significantly alter the drug development process and commercialize drug candidates by yielding safer drugs with enhanced therapeutic efficacy quickly and cost-effectively. With the increased demand for high-priced specialist therapies and safer drugs, the market for companion diagnostics has high growth potential. The growing importance of companion diagnostics also provides growth opportunities for the diagnostics segment and, in turn, the IVD reagents market.
CHALLENGES: Operational challenges in clinical process
Due to the rapid mutation of microbes and the increasing outbreak of epidemics, clinical laboratories need to adopt innovative technologies capable of rapid sample diagnosis. However, the shortage of skilled laboratory technicians to operate advanced diagnostic products has hindered overall adoption, particularly in emerging markets. In addition, a reluctance to move from manual operations toward automation is another challenge for market growth. Many providers find it difficult to transition to IT-based approaches from manual or traditional ones or may not prefer to make the shift.
Request Sample Report: https://www.marketsandmarkets.com/requestsampleNew.asp?id=131261429
Key Companies:
- Roche
- Danaher
- Abbott Laboratories
- Thermal Fisher
- Sysmex Corporation
- Biomerieux
- Siemens AG
- Ortho Clinical Diagnostics
- BD
- Bio Rad
- Myriad Genetics
- Hologic Inc
- Qiagen NV
- Mindray Medical
- Wondfo
- KHB
- Da An Gene
- Leadman
- BioSino
Recent Developments of IVD Reagents Market
- In September 2024, Thermo Fisher Scientific Inc. (US) introduced a testing/laboratory center for advanced lab services and instrumentation for pharma development worldwide.
- In December 2023, Danaher Corporation (US) acquired Abcam (UK) to grow its biological segment, which is critical for advancing drug discovery, life sciences research, and diagnostics.
- In December 2023, F. Hoffmann-La Roche Ltd (Switzerland) entered into a definitive agreement to acquire LumiraDx’s (US) point-of-care technology, thus combining multiple diagnostic modalities on a single platform.
- In May 2022, Hologic, Inc. (US) received CE marking for two new molecular assays, Panther Fusion EBV Quant Assay and Panther Fusion BKV Quant Assay, expanding its transplant pathogen monitoring menu on the Panther Fusion system.
Content Source:
https://www.marketsandmarkets.com/Market-Reports/ivd-reagents-market-131261429.html
https://www.marketsandmarkets.com/PressReleases/ivd-reagents.asp





















Comments
0 comment