views

The Potential of Cell Free Chromatin Inhibition in Cancer
Since 2017, seminal work by Dr. Mitra from Tata Medical Centre has been done on cell-free chromatin particles (cfChPs) that are released upon cell death. When cancer cells die, the skeletons of mutated cell-free chromatin may not always get buried in the tumor microenvironment but may escape the overwhelmed endogenous scavenging mechanisms and immune surveillance to roam about freely through the peripheral blood. During this time, they can wreak havoc by gaining entry into the houses of normal, healthy cells by integrating into their DNA and damaging it. They also inflict mitochondrial damage, enhance free radical (reactive oxygen species, ROS) production and perpetuate inflammation. It has been shown through preclinical and exploratory studies that cfChPs have the potential to enhance chemotherapy related toxicity and immune activation of cancer cells, thereby enhancing their survival.
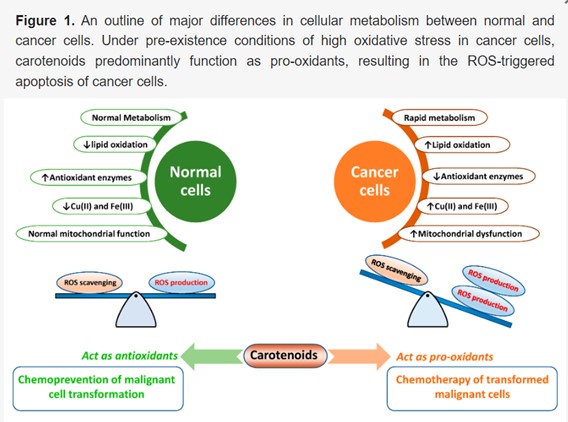
Oxidative stress due to free radicle generation leads to altered inflammation that then acts as a precancerous state of host cells, leading to the initiation of genetic mutations and impaired gene regulation. Antioxidants alone can remove free radicals and prevent tumorigenesis, but this must be balanced by prooxidant action to ensure damaged cells don’t benefit from the cell preserving (anti apoptotic) action of free radical clearance. This is how endogenous antioxidant mechanisms handle the dynamics of the onslaught that our bodies are subjected to, on a daily basis, but may become overwhelmed from time to time. In cancer cells, an innately high level of intracellular reactive oxygen species (ROS) exists, and the same antioxidants may act as potent pro-oxidant molecules and trigger ROS-mediated apoptosis. However, at least three factors can influence the function of an antioxidant, transforming it to a prooxidant; these factors include the presence of metal ions, the concentration of the antioxidant in matrix environments, and its redox potential, which forms the basis of exogenous interventions. The picture below describes the key differences between a normal and a cancer cell and the impact of the antioxidant-prooxidant function of carotenoids.
Dr. Mitra and colleagues have attempted inhibition of cfChPs with a prooxidant combination of resveratrol (an antioxidant) and copper in carefully selected doses, thus promising wide ranging therapeutic possibilities. The favorable biological consequences have recently made news through recent reports of exploratory and early phase clinical trials with the combination in oral cancer, gastric cancer and multiple myeloma. Resveratrol is a well-known polyphenolic plant derived antioxidant compound, and there are numerous studies associating it’s beneficial effects in cancer and in cardio- and neuro-protective effects5. In the combination, it reduces Cu(II) to Cu(I) generating reactive Oxygen species (ROS) and achieving DNA degrading activity at optimum concentrations desirable for anticancer activity6.
Such reverse engineering to curb the downstream effects of cancer cell death due to disease and drugs through down regulation of cancer hallmarks and immune checkpoints of cancer cells may yield far reaching benefits in ensuring a non-toxic, broadly applicable and affordable options for cancer patients.
If cancer cells appreciated a peaceful burial of their skeletons, in return for sparing misery to patients afflicted with cancer, then the handling of cfChP’s by this prooxidant combination would win their hearts. The results of investigations and later stage clinical trials are eagerly anticipated.
Read more: https://www.pharmafocusasia.com/articles/the-potential-of-cell-free-chromatin-inhibition-in-cancer
Follow us:





















Comments
0 comment