views
Origins of Tuberculosis Vaccination
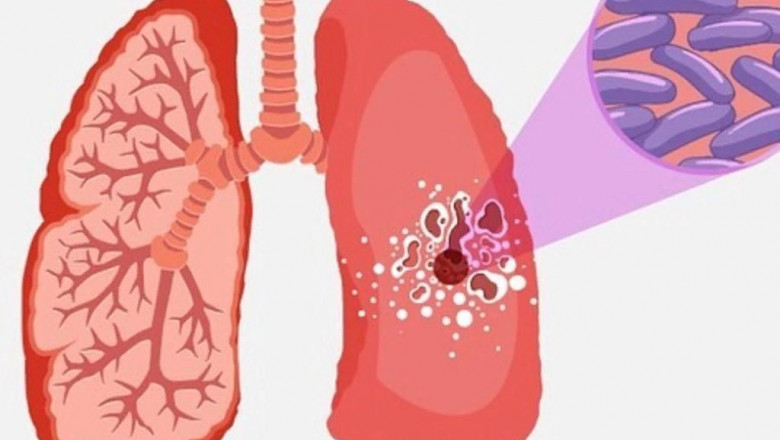
Tuberculosis (TB) has afflicted humans for thousands of years, with the earliest unambiguous evidence of the disease dating back 9,000 years. Caused by the bacterium Mycobacterium tuberculosis, TB most commonly attacks the lungs but can also affect other parts of the body such as the central nervous system, the lymphatic system, circulatory system, pleura, and bones. For much of history, there was no effective treatment for TB, which killed nearly one out of three people infected with the active form of the disease.
It was not until the late 19th century that scientists began developing the first TB vaccines. In 1890, French biologist Albert Calmette and French bacteriologist Camille Guérin initiated research that would span two decades and ultimately lead to the creation of the Bacille Calmette-Guérin (BCG) vaccine. Extracted from a weakened strain of the bovine tuberculosis bacterium Mycobacterium bovis, BCG represented a major breakthrough as the first viable vaccine against any human pathogen. Over the next several decades, clinical trials demonstrated BCG's ability to protect children from severe forms of TB such as tuberculous meningitis or military tuberculosis.
While effective, BCG's protective effects varied significantly by location. This raised questions about whether a more effective vaccine could be developed. In the mid-20th century, with Tuberculosis Vaccine on the rise globally due to factors like war, urbanization, and poor living conditions, vaccine research intensified in response to the urgent public health threat. Scientists explored new vaccine formulations and delivery methods in hopes of improving upon BCG's variable efficacy.
Modern Tuberculosis Vaccine Candidates
Since the 1970s, various tuberculosis vaccine candidates have emerged and been evaluated in clinical trials with the goal of developing a vaccine that provides stronger, longer-lasting immunity than BCG. Some notable vaccine candidates that have progressed to human efficacy testing include:
- MVA85A: A viral vector vaccine containing components of the M. tuberculosis antigen 85A, developed in the UK. Phase IIb trials showed no significant additional protective efficacy compared to BCG alone.
- ID93/GLA-SE: A subunit vaccine comprising four M. tuberculosis antigens combined with GLA-SE, an adjuvant developed by Immune Design. Phase IIa trials demonstrated good safety and immunogenicity. Phase IIb efficacy studies are ongoing.
- H4:IC31: A protein subunit vaccine containing two M. tuberculosis antigens combined with the IC31 adjuvant system, developed in Norway. Phase IIa trials found the vaccine well-tolerated and capable of boosting BCG-primed immune responses. Phase IIb efficacy trials have been proposed.
- M72/AS01E: A protein-adjuvant subunit vaccine from GlaxoSmithKline containing two M. tuberculosis antigens. Phase IIb trials showed no additional protective effect versus BCG alone, though post-hoc analysis indicated potential benefit in HIV-positive individuals.
While no vaccine candidate has yet proven sufficiently more protective than BCG in efficacy trials, researchers have gained useful knowledge on which antigens, delivery methods, and adjuvant systems hold promise. Ongoing phase IIb and III trials of ID93/GLA-SE and other candidates may provide clearer answers in the coming years on the potential for an improved TB vaccine.
Remaining Challenges in Tuberculosis Vaccine Development
Several major challenges still hinder development of a more effective tuberculosis vaccine to replace or boost BCG. Chief among these is the complex and incompletely understood nature of the host immune response required for protection against TB. Researchers have yet to identify an immunological "correlate of protection" that reliably predicts vaccine efficacy. Compounding this difficulty are the ability of M. tuberculosis to evade immune detection and persist latently in the body for decades without causing active disease.
Another challenge lies in evaluating new TB vaccine candidates. Traditional efficacy trials requiring very large sample sizes and long follow-up periods to detect differences in TB infection rates between vaccinated and control groups. Such studies present enormous logistical and financial hurdles. New study designs and biomarkers are being explored to streamline clinical development pathways.
Additional obstacles include the lack of animal models fully recapitulating human TB disease and latency. Limited understanding of how different populations like HIV-infected individuals might respond to vaccination also complicates vaccine design. Continued basic research into M. tuberculosis biology and pathogenesis remains vital for improving vaccine antigen selection and immunization strategies against this deadly infectious disease.
While no replacement for BCG appears imminent, steady progress is being made through joint global efforts in tuberculosis vaccine R&D. Upcoming trial results may help prioritize the most promising candidates suitable for specific patient groups or settings. With sustained commitment and innovation, the development of a more protective post-BCG vaccine remains a realistic long-term goal to curb the ongoing global tuberculosis epidemic.
Discover the language that resonates with you-
About Author-
Money Singh is a seasoned content writer with over four years of experience in the market research sector. Known for her strong SEO background, she skillfully blends SEO strategies with insightful content. Her expertise spans various industries, including food and beverages, biotechnology, chemical and materials, defense and aerospace, consumer goods, etc. (https://www.linkedin.com/in/money-singh-590844163)





















Comments
0 comment