views
Pharmacogenomics and disease
Pharmacogenomics is the science of study of genetic variability and its effects on the drug action. This determines the behavior of a drug inside the individual’s body. Every human being has different genetic makeup, due to which there is a difference in the overall metabolic activity so the action of drugs is also expected to be different in individuals, which is ultimately affecting the final outcome of the therapy. Applications of pharmacogenomics are limited to the theoretical concept till now and only focused on individualized drug therapy, although if explored to its full potential, it can change the entire therapeutic system with higher degree of accuracy, effectiveness and lowest level of adverse events [1]. Pharmacogenomics may have its applications beyond individualized drug therapy, such as diagnosis of disease, dosage formulation development and evaluation, targeted delivery systems, theranostic delivery systems and polymer activities [2]. Gene’s code for and control different biological activities (metabolic activities) inside the body via different intermediates such as enzymes, proteins, hormones, and effector cells. These mentioned intermediates may act as the diagnostic and therapeutic biomarkers and may help in diagnosis of disease and designing a suitable dosage form [3]. Identification of genome-based disease specific biomarkers (both diagnostic and therapeutic) is the first step in designing the suitable therapeutic system (fig 1).
In the area of cancer research, pharmacogenomics-based approaches of diagnosis and treatment could be of prodigious importance [4]. In cancer therapies, pharmacogenomics can be used to guide pharmacotherapy decisions, reduce adverse effects, and improve clinical outcomes. Cancer is a complex disease that involves the growth and spread of abnormal cells in the body. Cancer cells behave differently than normal cells because variations in receptors, biomolecules and biomarkers (either overexpressed or under expressed), hence, the basic requirements for the cancer cells to be alive is different in comparison to normal cells [5]. The treatment of cancer typically involves a combination of surgery, radiation therapy, and chemotherapy. Chemotherapy drugs work by targeting and killing rapidly dividing cancer cells, but they can also damage healthy cells in the process, leading to side effects. Targeted therapies are drugs that are designed to selectively kill cancer cells by identifying and targeting the specific proteins or pathways that are crucial for tumor growth. However, all patients do not respond to targeted therapies, and some may experience severe side effects [6]. But use of pharmacogenomics-based biomarkers in development of cancer therapy ensures the safety of normal cells via optimization of formulation characteristics, as each patient may vary depending on their genetics, age, weight, and other factors, so individualized optimization may be helpful [7] (fig. 2).
Therefore, along with the guiding pharmacotherapy decisions, pharmacogenomics can also help researchers to develop new cancer drugs that are more effectively and accurately targeted by understanding and identifying the genetic basis of cancer and new drug targets \while minimizing damage to healthy cells [8]. Genetic testing can help identify patients who have specific mutations or alterations in genes (diagnostic genomics) that are known to be targeted by the selected and specific drugs (treatment genomics). By selecting patients who are more likely to respond to treatment, clinicians can improve clinical outcomes and reduce the risk of adverse effects [9]. Genes such as BRCA1, BRCA2, TP53, EGFR, KRAS, HER, ALK, BRAF, PTEN, CDKN2A, RET, MLH1, MSH2, MSH6, PMS2 and VHL are found to have their role in the pathophysiology of different types of cancers
Mutation or polymorphism in the genes, as mentioned in above table 1, may be responsible for the risk of different types of cancers. Any mutation in these genes can be detected using different techniques such as next generation gene sequencing, multiplex ligation-dependent probe amplification (MLPA), Immunohistochemistry, fluorescence in situ hybridization (FISH) and others. The analysis of the gene mutation can be helpful in understanding the pathophysiology of disease and selection of suitable drug molecules, delivery route, delivery approach and dosage form to manage the cancer in an effective manner.
Pharmacogenomics and drug delivery
Knowledge of the pharmacogenomic-based interaction of cancer and cancer drugs can help in identification of suitable drugs for a specific type of cancer cells and further design and development of a suitable dosage forms that not only deliver the drug in optimal order but also improve the therapeutic outcome of cancer treatment by targeting the drugs to the specific site only. Genes control the synthesis of proteins, which further control the different physiological mechanisms of body including membrane permeability, transporter proteins, drug carriers, metabolizing enzymes, drug binding proteins, receptor sensitivity, receptor affinity, receptor confirmation and other cellular activities etc. Genes also controls the cellular functions and any alteration in genes may result in elevation or depression of factors (diagnostic biomarkers) that are directly responsible for cancer and involved in its pathophysiology. All these processes collectively affect the pharmacokinetics and pharmacodynamics of drug molecules inside the biological system [12]. Therefore, apart from finding new drug molecules, another approach could be delivering the old molecules with improved and optimized dosage forms. So, dosage formulations may need to be tailored for the individual patients based on their genetic profile. For example, if a patient has a genetic variation that affects how they metabolize a certain drug, a lower dose may be needed to avoid toxicity. Alternatively, a higher dose may be necessary for patients who have a genetic variation that affects how the drug is absorbed or distributed throughout the body [13].
Overall, pharmacogenomics has the potential to improve cancer treatment by providing personalized, more effective and safer therapies and optimized dosage forms as well (Fig. 1).
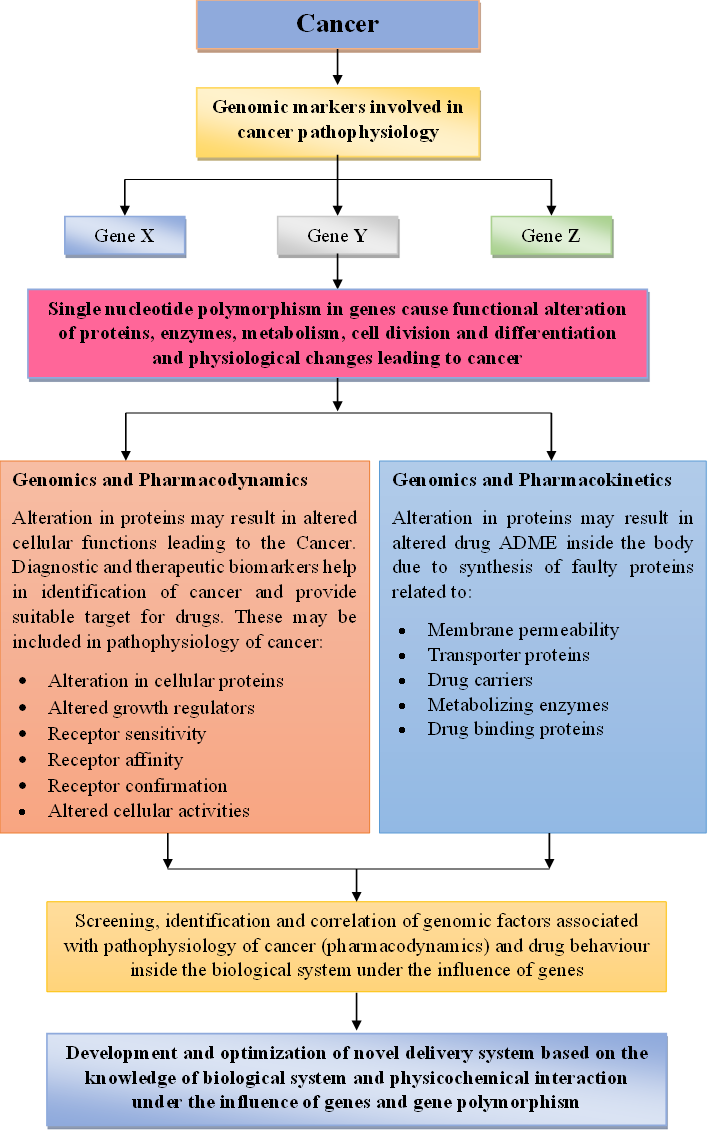
Fig 1: Role of pharmacogenomics in detection of cancer and development of new drugs for treatment

Fig 2: Graphical representation of correlation of cancer and pharmacogenomics in drug delivery optimization
Challenges
Pharmacogenomics, though promising for personalized cancer treatment, faces substantial obstacles. The intricate nature of cancer genetics, with its complex interactions and tumor heterogeneity, makes pinpointing relevant genes difficult. Access to comprehensive genomic profiling is limited by cost and interpretation of vast data requires expertise and sophisticated tools. Additionally, clinical validation of many approaches and uncertainties around reimbursement hinder widespread adoption. Ethical concerns surrounding privacy and discrimination, along with the need for better understanding of gene-drug interactions and integration with clinical workflows, further complicate the landscape. Despite these challenges, ongoing research, technological advancements, and regulatory reforms offer hope for overcoming them and unlocking the true potential of pharmacogenomics in revolutionizing cancer care.






















Comments
0 comment