views
Mycoplasma Testing Detection Methods
Mycoplasma testing is an important part of assuring the quality and safety of cell cultures used in research and biomanufacturing. Mycoplasma contaminations can negatively impact cell cultures used for biological drug production and scientific experimentation. There are various methods used for mycoplasma detection in cell cultures, each with their own advantages and limitations.
Culture-Based Detection Methods
Traditional culture-based mycoplasma detection relies on incubating a sample of the cell culture in mycoplasma-specific growth media. Any mycoplasmas present in the sample will grow out over a period of 10-21 days. Although widely considered the gold standard, culture-based methods are time-consuming and labor-intensive. The lengthy incubation period also means results are not available for 1-3 weeks, making the method unsuitable for process monitoring and quality control applications that require a rapid turnaround time.
DNA-Based PCR Detection
Polymerase chain reaction or PCR-based Mycoplasma Testing has largely replaced cell culture-based methods due to its high sensitivity, specificity and ability to provide results within days instead of weeks. In a PCR assay, mycoplasma DNA is extracted from the cell culture sample and amplified using genus- or species-specific primers. Detection is done through gel electrophoresis or probe-based methods like real-time quantitative PCR. While PCR is very accurate, it requires specialized equipment and training to perform which may not be available in all testing labs.
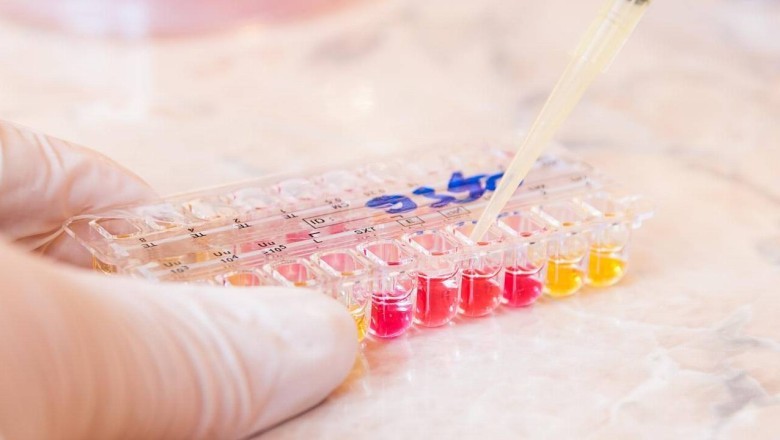
Microbiological Mycoplasma Testing
Alternative methods like microbiological assays utilize biochemical indicators and specialized growth media to detect changes caused by mycoplasma metabolism. One such assay uses the pH-sensitive dye phenol red – when mycoplasmas are present, they will metabolize nutrients in the media and change the pH resulting in a color change of the dye. Microbiological testing methods are simpler to perform than culture or PCR but tend to have reduced sensitivity. They serve as cheaper screening options but may require confirmatory testing using a more sensitive method.
DNA Microarray Platforms
DNA microarrays take advantage of PCR amplification and allow for identification of common mycoplasma species. In these assays, mycoplasma-specific DNA probes are spotted onto a surface and the amplified target DNA from the sample binds to complementary probes on the array. Microarrays enable detection of multiple mycoplasma species simultaneously and identification to be achieved within 24 hours. However, the technique has higher complexity and costs compared to PCR-based protocols.
Regulatory Requirements And Testing Frequency
Mycoplasma contamination of cell cultures is a significant issue impacting both scientific research and biopharmaceutical manufacturing. Regulatory agencies worldwide have established guidelines on routine mycoplasma testing to help mitigate the risks.
Compliance with Good Cell Culture Practice
Adherence to good cell culture practice (GCCP) principles is important to ensure reproducibility and reliability of cell-based experiments. GCCP standards require initial and routine mycoplasma testing of all cell cultures used for research and manufacturing purposes. Tests must be conducted using an appropriate method with documented verification of its performance characteristics.
FDA Process Validation Recommendations
The United States FDA provides extensive guidance for process validation of biopharmaceutical manufacturing. As per their recommendations, all cell banks and working cell culture stocks must undergo mycoplasma testing using an approved method before use. Testing is recommended at a minimum of every 6 months for cell banks in storage and every 2-3 months for cultures in active use like process development stages. Any positive findings will require corrective and preventive actions to be taken.
European Pharmacopoeia Standards
The European Pharmacopoeia has established mycoplasma testing specifications in their general chapters on "Microbiology" and "Cell Substrates for the Production of Vaccines for Human Use". All primary and working cell banks must be tested for the absence of mycoplasma using a validated culture or non-culture based detection method. Routine checks on cultures used in production are recommended at a stated periodicity depending on their application and risk level.
Importance of a Testing Program
Compliance with regulatory requirements helps assure product and data integrity but there are scientific necessities as well driving the need for regular mycoplasma screening. Contaminations are difficult to detect in early or low-level states and can disrupt cell function subtly over time. A robust and well-designed mycoplasma testing program protects research and manufacturing investments by facilitating early detection and mitigation of potential issues.
Mycoplasma Testing In Biopharmaceutical Production
Mycoplasma contamination poses serious risks to biologic drug manufacturing where cell cultures are used extensively as bioreactors for production of vaccines, monoclonal antibodies and other biotherapeutics
Testing During Process Development
During cell line and process development phases, mycoplasma screening assumes greater importance to avoid producing clinical materials from contaminated cell banks or cultures. Multiple testing points are often built into development timelines including testing of primary cells, cell banks and culture systems at amplification/passaging stages to confirm freedom from contamination proactively.
Even low-level contaminations can affect product quality attributes like high molecular weight aggregates, charge variation and protein glycosylation profiles. There are also safety concerns if live mycoplasma make their way into final drug products administered to patients.
Discover the Report for More Insights, Tailored to Your Language.
French German Italian Russian Japanese Chinese Korean Portuguese
Money Singh is a seasoned content writer with over four years of experience in the market research sector. Her expertise spans various industries, including food and beverages, biotechnology, chemical and materials, defense and aerospace, consumer goods, etc. (https://www.linkedin.com/in/money-singh-590844163)





















Comments
0 comment