views
Metabolic Stability - A Critical Aspect in Drug Discovery
Understanding metabolic stability helps to assess pharmacokinetic parameters for determining drug dose and frequency. Enhancement of the drug’s metabolic stability helps improve patient compliance concerning dosing and dose frequency. Measuring metabolic study by microsomal t1/2 approach combined with the automated LC-MS/MS and sample pooling strategies delivers reliable data with high-throughput screening.
The liability of a drug substance to the metabolism is referred to as metabolic stability [1]. The liver uses CYP-mediated metabolism to clear most orally administered drugs. Metabolic stability data is essential for evaluating and optimising the human therapeutic dose for the drug. In vitro systems obtained fromthe human liver, such as microsomes and hepatocytes, serve as dominant models to assess the metabolic stability of the drug [2-4]. Human liver microsomes offer numerous advantages, i.e., such as ease of availability and use for high-throughput screens but lack phase II metabolising enzymes, unlike hepatocytes [5, 6].Predicting in vivo pharmacokinetic data using in vitro drug metabolism data in preclinical investigations is of tremendous relevance. The in vitro metabolism data aids in selecting promising leads for further research. Drug clearance gives information on the dose required for maintaining steady-state plasma concentration and drug disposition [7, 8].
2. Significance of metabolic stability in drug design
The drug discovery and development processrelies on pharmacokinetics and metabolism studies. Pharmacokinetics and metabolism data from animal studies offer trustworthy physiologically relevant information [9]. In vitro studies are preferred as the in vivo studies are time-consuming and more expensive. In vitro drug metabolism studies with high-throughput screening have become more popular as evidence of a high link between in vitro and in vivo metabolic data has grown [10]. Drug discovery isstreamlined to be a more precise, fast, and iterative process. It is a continuous and iterative process to improve the structural scaffolds of drugs by medicinal chemists for identifying the ideal molecule. Maximum output is achieved by quickly generating and interpreting data from the metabolism studies for the designed drug. Knowledge of metabolic soft spots in a drug structure is crucial at the lead identification stage. This insight enables them to make structural alterations in the lead optimisation process to improve metabolomic stability. Further, it will be convenient to gather information on the impact of various structural modifications on metabolic stability during the tuning of pharmaceutical activity [11].
3. Approaches to improve the metabolic stability
The key to optimum drug design is to identify the best combination of several properties. Improvement of metabolic stability results in the enhanced plasma half-life of the drug substance. Moreover, it is essential to assess critical parameters such as elimination or reducing first-pass metabolism, enzyme induction, enzyme inhibition, and reactive or toxic metabolites [12]. Figure 1 illustrates the strategies to enhance the metabolic stability of a drug substance. In general, increasing the hydrophilicity or polarity by incorporating isosteric groups and blocking or replacing metabolic soft spots with other stable groups of the drug substances enhances metabolic stability[13]. Decreasing the drug’s lipophilicity reduces favourable binding contact with the CYP enzymes due to reduced hydrophobic interactions, decreasing the drug’s metabolism. In addition, the polarity of the molecule can also be increased by incorporating the heteroatom in the benzene ring, e.g., benzene to more polar pyridine.
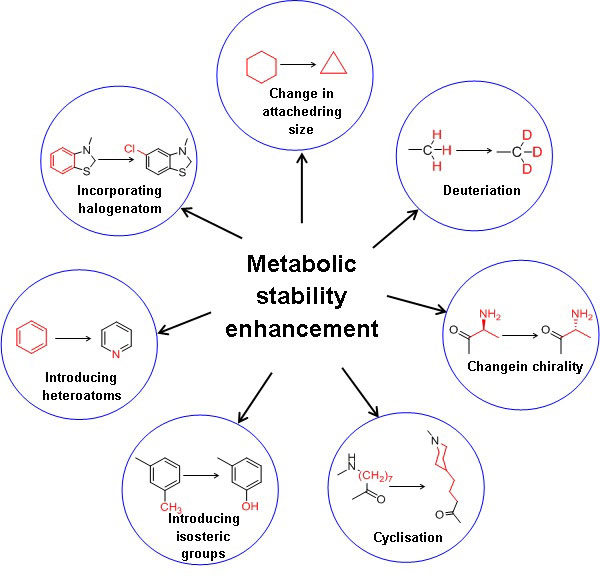
Deuterium incorporation at the potential metabolic soft spot is a novel strategy for enhancing metabolic stability with the same biological activity as the parent drug. The addition of deuterium to the molecule also affects the drug’s metabolic liabilities. The carbon deuterium bond is shorter on the order of 0.005 and stronger relative to the carbon-hydrogen bond due to the anharmonicity single bond vibrations.
Read more: https://www.pharmafocusasia.com/articles/metabolic-stability






















Comments
0 comment