views

In the dynamic landscape of pharmaceutical research, small- to medium-sized biotech firms, many of which operate virtually, play a crucial and integral role in early drug development. These firms focus on reaching key milestones, such as submitting investigational new drug (IND) applications or leveraging intellectual property for acquisition deals. Given their unique goals, constraints, and regulatory requirements, their approach to Chemistry, Manufacturing, and Controls (CMC) must be highly customized. The strategic use of external advisors and contract manufacturing organizations (CMOs) is critical in crafting CMC strategies tailored to each company's needs.
Transitioning from discovery to market is a complex journey, with only about 6% of molecules progressing to Phase III clinical trials. This high early attrition rate, often due to CMC complexities, underscores the need for strategic planning to navigate the CMC landscape from development to commercialization effectively. A well-thought-out strategy can provide reassurance and confidence in facing these challenges. The rapid development of COVID-19 vaccines, achieved in less than a year, serves as a beacon of hope, demonstrating the potential to expedite development phases while upholding safety and compliance standards.
The 'Fastlane' strategy, designed to reduce market entry times, involves proactive planning and resource allocation to promising drug candidates. However, the need for speed must be carefully balanced with the responsibility to ensure patient safety and regulatory compliance. Diligent attention to these aspects is crucial to minimize financial risks and enhance the likelihood of clinical success (Figure 1).
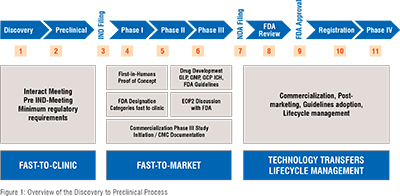
NAVIGATING FDA GUIDELINES FOR RAPID CLINICAL ENTRY:
The FDA's five special designations—Orphan, Fast Track, Accelerated Approval, Priority Review, and Breakthrough Therapy—accelerate the development and approval of medications for severe medical conditions with unmet needs, benefiting healthcare providers and patients. Orphan Designation targets drugs for rare diseases affecting fewer than 200,000 people in the U.S., offering benefits like tax credits and extended exclusivity. Fast Track facilitates faster development for severe conditions lacking therapies by enabling more frequent FDA interactions and a rolling review process. Accelerated Approval allows drugs that improve on existing treatments through surrogate endpoints to enter the market sooner, pending confirmation in post-marketing studies. Priority Review shortens FDA review times from ten months to six for drugs with significant treatment advances, while Breakthrough Therapy offers all Fast Track benefits plus intensive FDA guidance for drugs that markedly improve on existing therapies, ensuring rapid and efficient market entry.
OPTIMIZING IND SUBMISSIONS FOR FAST-TRACK APPROVAL
The requirements for an Investigational New Drug (IND) submission are critical in the fast-to-clinic approach, demanding a comprehensive dossier that aligns with the strict regulatory standards of the U.S. Food and Drug Administration (FDA). This dossier includes detailed preclinical study data such as pharmacological, toxicological, and pharmacokinetic evaluations to affirm the investigational product’s safety and justify human trials [8, 9]. It also incorporates Chemistry, Manufacturing, and Controls (CMC) information detailing the synthesis, characterization, and quality control of the drug substance and product, ensuring their consistency and safety. Moreover, the submission must present a proposed clinical trial protocol that outlines the study design, objectives, and methodology, along with credentials of clinical investigators and informed consent forms for participants, making it a pivotal step in advancing to clinical trials.
Discover more: https://www.pharmafocusamerica.com/strategy/driving-biotech-innovation






















Comments
0 comment