views
Plaque Psoriasis Market Projected to Grow with Biologic Therapies
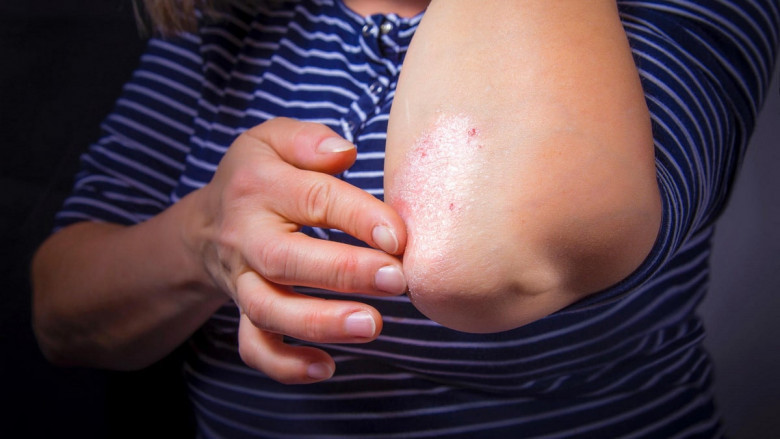
Plaque psoriasis is a chronic autoimmune skin disorder characterized by red, inflamed patches covered with silvery scales. Therapeutic products for plaque psoriasis include topical corticosteroids, vitamin D analogues, systemic agents, and the latest biologic therapies such as IL-17 and IL-23 inhibitors. Biologic treatments offer targeted modulation of the immune response, minimizing systemic side effects and delivering faster, more durable clearance of psoriatic lesions.
The adoption of self-injectable pens and prefilled syringes enhances patient convenience, adherence, and overall treatment satisfaction. Rising patient awareness, improvements in diagnostic methods, and robust clinical trial data have collectively expanded the treatment landscape. Furthermore, healthcare providers are increasingly leveraging Plaque Psoriasis Market research and real-world evidence to optimize treatment algorithms, reduce hospital visits, and improve long-term patient outcomes. The integration of teledermatology platforms has also played a crucial role in remote patient monitoring and timely intervention.
The plaque psoriasis market is estimated to be valued at USD 32.28 Bn in 2025 and is expected to reach USD 52.17 Bn by 2032, growing at a compound annual growth rate (CAGR) of 7.1% from 2025 to 2032.
Key Takeaways
Key players operating in the Plaque Psoriasis Market are:
-AbbVie Inc.
-Novartis International AG
-Johnson & Johnson (Janssen Pharmaceuticals)
-Eli Lilly and Company
-Amgen Inc.
With growing unmet needs, the market opportunities lie in expanding access to advanced biological treatments in emerging economies. Increased healthcare spending, favorable reimbursement policies, and government initiatives to improve dermatological care are opening new market segments. Additionally, strategic collaborations between pharmaceutical companies and research institutes are expected to drive innovation in next-generation biologics and small molecule inhibitors. Market analysis indicates that combination therapies and personalized medicine approaches hold significant promise for addressing moderate to severe cases, thereby broadening the market size and driving sustainable market growth.
The rapid proliferation of biologic therapies represents a key technological advancement transforming treatment paradigms. Cutting-edge innovations such as monoclonal antibodies targeting TNF-α, IL-17, and IL-23 have demonstrated superior efficacy and safety profiles in clinical trials. The integration of biomarkers and genomic profiling tools is enhancing patient stratification and treatment customization. Telehealth platforms, mobile health apps, and electronic patient-reported outcome systems are reshaping patient engagement and adherence. These advancements are poised to redefine market dynamics, support robust market insights, and steer future market trends toward precision dermatology.
Market drivers
One of the primary market drivers for the plaque psoriasis market is the rising prevalence of autoimmune skin disorders worldwide. According to epidemiological studies, the global incidence of plaque psoriasis continues to climb due to genetic predisposition, environmental factors, and lifestyle changes. This growing disease burden is fueling demand for innovative treatment options and driving market growth. Enhanced diagnostic capabilities, including dermoscopy and digital imaging, enable early disease detection and prompt initiation of therapy, further propelling market size. Additionally, supportive regulatory frameworks and expedited approval pathways for breakthrough biologics have accelerated product launches. Increasing investments by key market players in clinical trials and research-and-development activities are expanding the product pipeline, thus reinforcing market drivers. Combined with rising patient awareness of effective therapies, these factors collectively contribute to a favorable market forecast, robust market revenue expansion, and sustained business growth throughout the forecast period.
Current Challenges in the Plaque Psoriasis Industry
The plaque psoriasis sector faces several complex obstacles that hinder sustained growth and innovation. One of the primary hurdles stems from the variability in patient response to biologic therapies, which complicates treatment protocols and increases the burden on healthcare providers. Furthermore, stringent regulatory requirements and lengthy approval timelines delay the introduction of new therapeutic agents, adding to development costs and risking investor confidence. Supply chain disruptions—exacerbated by global events—also introduce volatility in the availability of critical biologics and delivery mechanisms. High treatment costs and inconsistent reimbursement policies across regions limit patient access and impact the overall industry landscape. Moreover, ongoing patent expirations expose incumbent players to biosimilar competition, intensifying pricing pressures and reducing profit margins. Despite robust market research and significant R&D investments, there remains an unmet need for more targeted treatments with improved safety profiles. Addressing these hurdles demands a refined understanding of evolving market trends and patient subgroups, as well as enhanced collaboration between manufacturers, clinicians, and payers. By acknowledging these gaps, stakeholders can leverage core market drivers and emerging technologies—such as precision medicine and digital health solutions—to capitalize on future market opportunities and increase market share.
SWOT Analysis
Strength:
A robust R&D pipeline underpinned by cutting-edge immunology insights has accelerated discovery of novel biologic agents. Strong intellectual property protection and well-established commercial channels ensure global reach and sustained brand recognition.
Weakness:
High manufacturing complexity and steep development costs limit accessibility for smaller innovators. Heterogeneity in patient subtypes complicates clinical trial design and personalization of therapy, leading to longer timeframes before broad adoption.
Opportunity:
Expansion into underserved regions with rising healthcare investments creates new entry points for innovative treatments. Integration of digital health platforms and companion diagnostics can improve adherence and offer real-world evidence to guide targeted therapy decisions.
Threats:
Emergence of lower-cost biosimilars threatens pricing structures and erodes margins for originator products. Regulatory uncertainties in emerging markets and shifting reimbursement models may delay market entry and constrain revenue forecasts.
Geographical Regions with Value Concentration
Established markets across North America and Western Europe account for the largest share of overall plaque psoriasis expenditure. These regions benefit from advanced healthcare infrastructure, high physician awareness, and comprehensive insurance coverage that supports premium pricing. Major urban centers in the United States and Germany host leading research institutions and manufacturing sites, creating robust regional value pools. Japan also commands a significant portion of revenue due to favorable regulatory frameworks and high patient demand for innovative therapies. In these territories, well-defined treatment guidelines and strong partnerships between industry and payers drive steady uptake of both first-line biologics and emerging small molecules. As a result, these areas remain focal points for clinical trial activity, regulatory submissions, and post-marketing surveillance, reinforcing their dominance in terms of overall financial commitment.
Fastest Growing Region
The Asia-Pacific corridor is emerging as the fastest expanding territory for plaque psoriasis therapies. Rapid urbanization, rising incomes, and increased public health spending have accelerated treatment adoption in China, India, and South Korea. Growing awareness among dermatologists, coupled with more accessible reimbursement schemes, fuels patient uptake of advanced biologics. Local clinical trial networks are expanding their scope, encouraging accelerated regulatory approvals for new agents. Additionally, partnerships between global manufacturers and regional distributors optimize supply chains and improve affordability. Southeast Asian nations—led by Malaysia and the Philippines—are also seeing notable growth as governments implement national psoriasis registries and educate healthcare professionals. Collectively, these dynamics position the Asia-Pacific region to outpace traditional markets, driven by favorable demographics, improving medical infrastructure, and heightened public-private collaboration.
‣ Get this Report in Japanese Language: 尋常性乾癬市場
‣ Get this Report in Korean Language: 플라크건선시장
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)










