views
AI in Clinical Trials Market Size and Growth
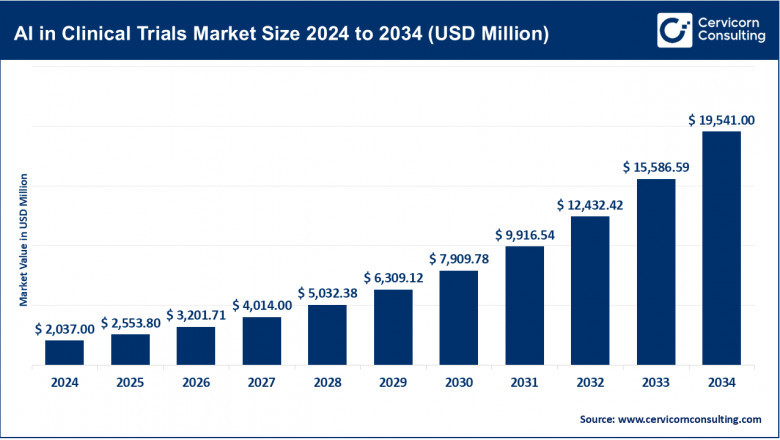
The global AI in clinical trials market size was valued at USD 2,037 million in 2024 and is projected to reach USD 19,541 million by 2034, registering a CAGR of 25.4% during the forecast period. This growth underscores AI’s increasing role in improving patient outcomes, reducing trial costs, and accelerating time-to-market for new therapies.
Why AI in Clinical Trials Matters
Clinical trials are the backbone of drug development, but they face a multitude of challenges:
- High Costs: A single trial can cost hundreds of millions of dollars.
- Low Success Rates: Less than 12% of drugs entering clinical trials receive FDA approval.
- Time-Intensive Processes: Trials can last from several months to over a decade.
- Recruitment Challenges: Identifying and enrolling the right patients is difficult and time-consuming.
AI addresses these issues by introducing automation, predictive analytics, and real-time monitoring capabilities that streamline each phase of the clinical trial lifecycle—from protocol design and patient recruitment to monitoring and data analysis.
Get a Free Sample: https://www.cervicornconsulting.com/sample/2635
Key Market Drivers
1. Growing Demand for Accelerated Drug Discovery
AI enables pharmaceutical companies to rapidly analyze clinical and molecular data, making it easier to identify potential drug candidates and predict clinical trial outcomes. Machine learning algorithms can simulate thousands of variables to optimize trial protocols and improve decision-making.
For instance, deep learning tools can identify biomarkers for targeted therapies or suggest optimal dosage levels—drastically cutting down trial phases and increasing the chances of success.
2. Rising Prevalence of Chronic and Complex Diseases
The global burden of diseases like cancer, cardiovascular conditions, neurological disorders, and autoimmune diseases is pushing pharmaceutical companies to innovate faster. AI provides the tools to manage this complexity—especially in identifying suitable trial participants and monitoring disease progression using real-time data from electronic health records (EHRs), genomics, and wearable devices.
3. Shift Toward Decentralized and Virtual Trials
Decentralized Clinical Trials (DCTs) gained traction during the COVID-19 pandemic, and AI is a major enabler of this model. By integrating with mobile health platforms, wearables, and telemedicine tools, AI allows remote patient monitoring, automated data capture, and seamless communication between stakeholders.
This decentralization not only reduces costs but also enhances patient diversity and trial accessibility, a known issue in conventional models.
4. Regulatory Support and Digital Health Initiatives
Governments and regulatory bodies are recognizing the potential of AI in clinical research. The U.S. FDA’s Digital Health Center of Excellence and Europe’s Horizon 2020 have laid the groundwork for AI adoption in drug development. Additionally, increased collaboration between regulators and technology providers is helping establish guidelines and frameworks for ethical AI use.
Regional Landscape
North America: Market Leader
North America dominates the AI in clinical trials market, with a 2024 valuation of USD 811.54 million, owing to strong R&D investments, a robust AI ecosystem, and regulatory initiatives that encourage innovation. Major pharmaceutical and biotechnology firms headquartered in the U.S. are at the forefront of AI deployment, especially in oncology and rare disease trials.
Europe: Emphasis on Precision Medicine
Europe accounted for USD 532.06 million in 2024, bolstered by its strong life sciences industry and growing focus on precision medicine. The European Medicines Agency (EMA) is supportive of AI applications, and GDPR compliance is accelerating innovation in privacy-preserving AI technologies, such as federated learning and differential privacy.
Asia-Pacific: Fastest-Growing Market
With a projected value of USD 4.86 billion by 2034, the Asia-Pacific region is the fastest-growing geography in this sector. China, India, Japan, and South Korea are increasing their investments in AI for healthcare, driven by government support, cost-effective clinical research environments, and expanding digital health infrastructure.
Key Applications of AI in Clinical Trials
- Patient Recruitment & Retention
- AI scans EHRs and social media to identify eligible patients faster than traditional methods.
- Predictive modeling anticipates dropouts and helps design interventions to retain participants.
- Protocol Optimization
- Natural Language Processing (NLP) analyzes historical trial data and medical literature to improve trial designs.
- AI simulations model different protocols to determine the most effective ones.
- Site Selection
- Algorithms identify optimal trial sites based on past performance, demographics, and geographic feasibility.
- This reduces delays and increases data quality.
- Real-Time Monitoring & Adverse Event Prediction
- AI-powered platforms continuously monitor patient vitals via wearables, predicting adverse events before they occur.
- Enables proactive interventions and improves patient safety.
- Data Management & Outcome Prediction
- Machine learning cleans, integrates, and analyzes multimodal datasets (text, images, signals).
- Predictive analytics helps forecast treatment responses, potentially shortening trial timelines.
Competitive Landscape
Leading players in the AI in clinical trials market are leveraging strategic partnerships, acquisitions, and product innovations to stay ahead.
Notable Companies & Initiatives
- Pfizer: Used AI in conjunction with BioNTech to accelerate COVID-19 vaccine development, particularly in trial planning and monitoring.
- Medable: A pioneer in DCTs, Medable uses AI to enhance patient onboarding and remote monitoring.
- Science 37: Integrates AI to scale virtual trials globally.
- TEMPUS & ConcertAI: Specialize in oncology trials, using real-world evidence and AI analytics for patient matching and cohort analysis.
- NetraMark & AiCure: Focus on behavioral data, using AI to detect anomalies in patient behavior and adherence.
These firms are redefining clinical trial norms, making trials more predictive, inclusive, and efficient.
Challenges and Ethical Considerations
While the potential of AI in clinical trials is vast, several challenges need to be addressed:
- Data Privacy & Security: Ensuring the integrity and privacy of sensitive health data, especially when using AI algorithms that require large-scale patient datasets.
- Bias in Algorithms: AI models trained on biased or non-representative data can lead to skewed outcomes, affecting patient safety.
- Regulatory Uncertainty: Lack of standardized global frameworks on AI use in clinical trials can slow down innovation.
- Interoperability: Integrating AI tools across diverse clinical and hospital systems remains a technical hurdle.
Solving these issues will be critical for long-term scalability and trust.
Future Outlook
Looking ahead, the convergence of AI with big data, genomics, cloud computing, and blockchain is expected to create a fully digitized, patient-centric ecosystem for drug development.
Key trends to watch include:
- Use of synthetic control arms to reduce placebo use in trials.
- Generative AI to simulate trial outcomes based on minimal real-world data.
- Federated learning to train AI models on decentralized data while maintaining privacy.
- AI-driven adaptive trial designs that modify parameters in real time based on interim results.
By 2034, AI is expected to become a standard component of every clinical trial phase, redefining the benchmarks of speed, accuracy, and patient safety.





















Comments
0 comment