Klap: The AI Video Editing Tool Revolutionizing Social Media Content Creation
-

Experience trusted Micro Needling Treatment in Whangaparaoa at Skin Renew.

GRS Certificate verifies recycled content and responsible practices. Ideal...

According to Fortune Business Insights Asia air conditioner market size was...

What if everything you thought you knew about executive presence was outdat...

Contact B&R Moll for information about our commercial die cutting machi...

Is your business focusing on technological solutions that align with the re...

Sha International Spa and Wellness is Best Spa in Chandigarh, offering rela...
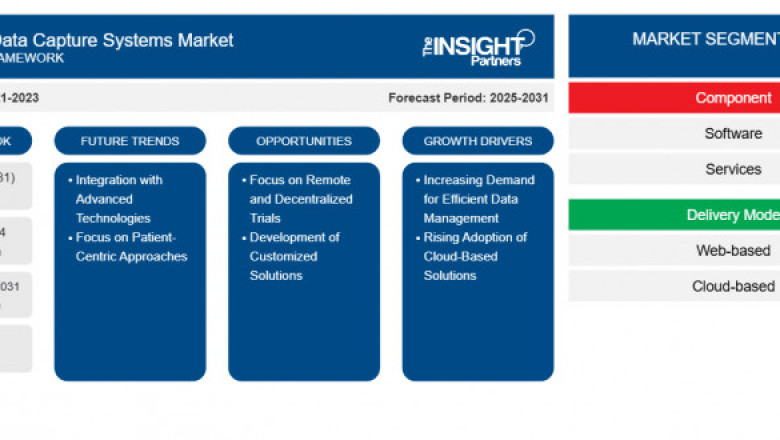
The EDC Systems market is witnessing rapid transformation with a surge in d...
