Mitolyn Ingredients Label
-


In the evolving digital landscape, entertainment applications have taken a...

24/25 Barcelona Home Coldplay Jersey
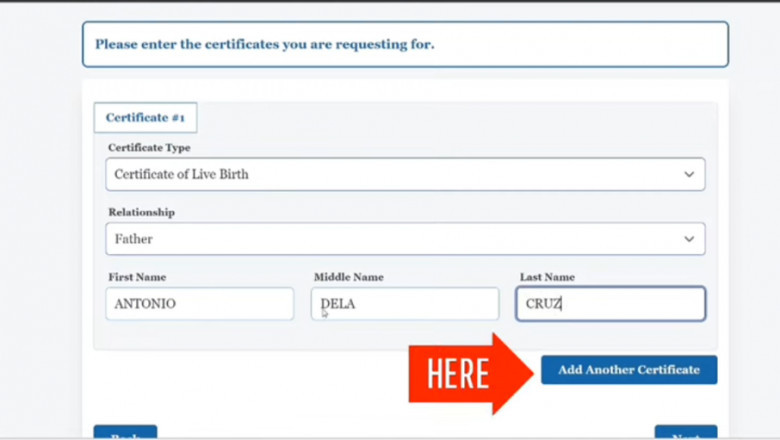
Booking a PSA appointment in New York has never been easier. This simple gu...

Shop online for Balloon Bouquets, Party Balloons, Personalised Balloons and...

With high-end features like foot rollers, 5D customizable massage technolog...

Maximize small spaces with a single size mattress—perfect for kids, guests,...

The Lighthouse is a foundation that is dedicated in providing a quality tre...

Ashirwad Vastu empowers individuals worldwide through expert Vastu Shastra...

